Ann Pediatr Endocrinol Metab.
2021 Sep;26(3):185-191. 10.6065/apem.2040220.110.
Clinical findings influencing time to menarche post gonadotropin-releasing hormone agonist therapy in central precocious puberty
- Affiliations
-
- 1Department of Pediatrics, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- 2Department of Pediatric Endocrinology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- KMID: 2520511
- DOI: http://doi.org/10.6065/apem.2040220.110
Abstract
- Purpose
This study aimed to evaluate the time interval to menarche after gonadotropin-releasing hormone agonist (GnRHa) treatment in females with central precocious puberty (CPP) and to identify factors contributing to timing of menarche.
Methods
We retrospectively reviewed medical records of 39 females with CPP who reached menarche after GnRHa treatment (leuprolide or histrelin). CPP diagnostic criteria were breast development at <8 years old, measurable pubertal luteinizing hormone and/or estradiol concentrations, and bone age advancement. Indications to treat were advanced bone age and psychosocial concerns. Descriptive summaries were reported as frequency and proportion for categorical variables and mean and standard deviation for continuous measures. Linear regression models were developed to evaluate the associations of clinical factors with the time interval to menarche.
Results
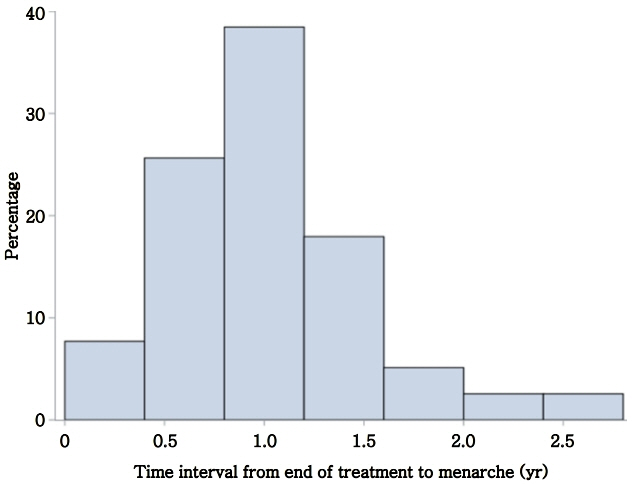
Mean age was 9.4±1.6 years at treatment onset, and treatment duration was 2.2±1.4 years. Menarche occurred at 12.6±1.1 years, which was 1.04±0.5 years after treatment discontinuation. This was negatively associated with Tanner stage of breast development and bone age at treatment onset and change in bone age during treatment. No association was seen between time interval to menarche and treatment duration, medication, or body mass index.
Conclusion
We found the average time interval to menarche after GnRHa treatment in our population of female patients with CPP to be 1.04±0.5 years; this is in agreement with other reports. Tanner stage of breast development, bone age at treatment onset, and change in bone age were negatively associated with time interval to menarche. These data provide clinical correlates that assist providers during anticipatory guidance of patients with CPP after GnRHa treatment.
Figure
Reference
-
References
1. Rosenfield RL, Cooke DW, Radovick S. Puberty and its disorders in the female. In : Sperling MA, editor. Pediatric endocrinology. 4th ed. Philadelphia (PA): Elsevier/ Saunders;2014. p. 569–663. e1.2. Lee PA. Central precocious puberty. An overview of diagnosis, treatment, and outcome. Endocrinol Metab Clin North Am. 1999; 28:901–18. xi.3. Carel JC, Lahlou N, Roger M, Chaussain JL. Precocious puberty and statural growth. Hum Reprod Update. 2004; 10:135–47.
Article4. Brito VN, Spinola-Castro AM, Kochi C, Kopacek C, Silva PC, Guerra-Junior G. Central precocious puberty: revisiting the diagnosis and therapeutic management. Arch Endocrinol Metab. 2016; 60:163–72.
Article5. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, Antoniazzi F, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009; 123:e752–62.
Article6. Baek JW, Nam HK, Jin D, Oh YJ, Rhie YJ, Lee KH. Age of menarche and near adult height after long-term gonadotropin-releasing hormone agonist treatment in girls with central precocious puberty. Ann Pediatr Endocrinol Metab. 2014; 19:27–31.
Article7. Gillis D, Karavani G, Hirsch HJ, Strich D. Time to menarche and final height after histrelin implant treatment for central precocious puberty. J Pediatr. 2013; 163:532–6.8. Neely EK, Lee PA, Bloch CA, Larsen L, Yang D, MattiaGoldberg C, et al. Leuprolide acetate 1-month depot for central precocious puberty: hormonal suppression and recovery. Int J Pediatr Endocrinol. 2010; 2010:398639.
Article9. Tanaka T, Niimi H, Matsuo N, Fujieda K, Tachibana K, Ohyama K, et al. Results of long-term follow-up after treatment of central precocious puberty with leuprorelin acetate: evaluation of effectiveness of treatment and recovery of gonadal function. The TAP-144-SR Japanese Study Group on Central Precocious Puberty. J Clin Endocrinol Metab. 2005; 90:1371–6.
Article10. Paterson WF, McNeill E, Young D, Donaldson MD. Auxological outcome and time to menarche following longacting goserelin therapy in girls with central precocious or early puberty. Clin Endocrinol (Oxf). 2004; 61:626–34.
Article11. Fuqua JS. Treatment and outcomes of precocious puberty: an update. J Clin Endocrinol Metab. 2013; 98:2198–207.
Article12. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002; (246):1–190.13. Greulich W, P yle S. R adiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford (CA): Stanford University Press;1959.14. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952; 40:423–41.
Article15. Heger S, Partsch CJ, Sippell WG. Long-term outcome after depot gonadotropin-releasing hormone agonist treatment of central precocious puberty: final height, body proportions, body composition, bone mineral density, and reproductive function. J Clin Endocrinol Metab. 1999; 84:4583–90.
Article16. Lee HS, Yoon JS, Park KJ, Hwang JS. Increased final adult height by gonadotropin-releasing hormone agonist in girls with idiopathic central precocious puberty. PLoS One. 2018; 13:e0201906.
Article17. Arrigo T, De Luca F, Antoniazzi F, Galluzzi F, Iughetti L, Pasquino AM, et al. Menstrual cycle pattern during the first gynaecological years in girls with precocious puberty following gonadotropin-releasing hormone analogue treatment. Eur J Pediatr. 2007; 166:73–4.
Article18. Mrug S, Elliott MN, Davies S, Tortolero SR, Cuccaro P, Schuster MA. Early puberty, negative peer influence, and problem behaviors in adolescent girls. Pediatrics. 2014; 133:7–14.
Article19. Schoelwer MJ, Donahue KL, Bryk K, Didrick P, Berenbaum SA, Eugster EA. Psychological assessment of mothers and their daughters at the time of diagnosis of precocious puberty. Int J Pediatr Endocrinol. 2015; 2015:5.
Article20. Menk TAS, Inácio M, Macedo DB, Bessa DS, Latronico AC, Mendonca BB, et al. Assessment of stress levels in girls with central precocious puberty before and during long-acting gonadotropin-releasing hormone agonist treatment: a pilot study. J Pediatr Endocrinol Metab. 2017; 30:657–62.
Article21. Mercader-Yus E, Neipp-López MC, Gómez-Méndez P, Vargas-Torcal F, Gelves-Ospina M, Puerta-Morales L, et al. Anxiety, self-esteem and body image in girls with precocious puberty. Rev Colomb Psiquiatr. 2018; 47:229–36.
Article22. Bereket A. A critical appraisal of the effect of gonadotropinreleasing hormon analog treatment on adult height of girls with central precocious puberty. J Clin Res Pediatr Endocrinol. 2017; 9:33–48.
Article23. Lazar L, Padoa A, Phillip M. Growth pattern and final height after cessation of gonadotropin-suppressive therapy in girls with central sexual precocity. J Clin Endocrinol Metab. 2007; 92:3483–9.
Article24. Klein KO, Dragnic S, Soliman AM, Bacher P. Predictors of bone maturation, growth rate and adult height in children with central precocious puberty treated with depot leuprolide acetate. J Pediatr Endocrinol Metab. 2018; 31:655–63.
Article25. Micillo M, Salerno M, Officioso A, Perna E, Gasparini N, Pisaturo L, et al. Near final height after GnRH agonist treatment in central precocious puberty. J Pediatr Endocrinol Metab. 2000; 13 Suppl 1:787–90.
Article26. Pasquino AM, Pucarelli I, Accardo F, Demiraj V, Segni M, Di Nardo R. Long-term observation of 87 girls with idiopathic central precocious puberty treated with gonadotropinreleasing hormone analogs: impact on adult height, body mass index, bone mineral content, and reproductive function. J Clin Endocrinol Metab. 2008; 93:190–5.
Article27. Jung MK, Song KC, Kwon AR, Chae HW, Kim DH, Kim HS. Adult height in girls with central precocious puberty treated with gonadotropin-releasing hormone agonist with or without growth hormone. Ann Pediatr Endocrinol Metab. 2014; 19:214–9.
Article28. Korkmaz O, Sari G, Mecidov I, Ozen S, Goksen D, Darcan S. The gonadotropin-releasing hormone analogue therapy may not impact final height in precocious puberty of girls with onset of puberty aged 6 - 8 years. J Clin Med Res. 2019; 11:133–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Indication of Gonadotropin-Releasing Hormone Agonist: Precocious Puberty or Advanced Puberty?
- Age of menarche and near adult height after long-term gonadotropin-releasing hormone agonist treatment in girls with central precocious puberty
- Therapy for idiopathic precocious puberty in children
- Proper Dosage and Duration of GnRH Agonist Treatment in Central Precocious Puberty
- Two Cases of Secondary Central Precocious Puberty in Boys with Congenital Adrenal Hyperplasia - before and after Treatment with Corticosteroid