J Rheum Dis.
2021 Oct;28(4):216-224. 10.4078/jrd.2021.28.4.216.
The TNF-NF-κB-DKK1 Axis Promoted Bone Formation in the Enthesis of Ankylosing Spondylitis
- Affiliations
-
- 1Hanyang University Institute for Rheumatology Research, Seoul, Korea
- 2Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea
- 3Department of Translational Medicine, Graduate School of Biomedical Science and Engineering, Hanyang University, Seoul, Korea
- 4Department of Orthopedic Surgery, Hanyang University Hospital, Seoul, Korea
- 5Department of Orthopedic Surgery, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- KMID: 2520434
- DOI: http://doi.org/10.4078/jrd.2021.28.4.216
Abstract
Objective
This study aimed to determine the serum Dickkopf 1 (DKK1) levels in ankylosing spondylitis (AS) patients and decipher the mechanism of tumor necrosis factor (TNF)-mediated DKK1 regulation in human AS enthesis cells.
Methods
The sera were obtained from 103 patients with AS and 30 healthy controls (HCs). The enthesis of facet joints were obtained from 4 AS patients and 5 controls. The serum levels of DKK1 were measured using ELISA and compared between AS and HCs. The impact of TNF on DKK1 expression in human primary spinal enthesis cells was evaluated using various molecular biology techniques and bone formation indicators.
Results
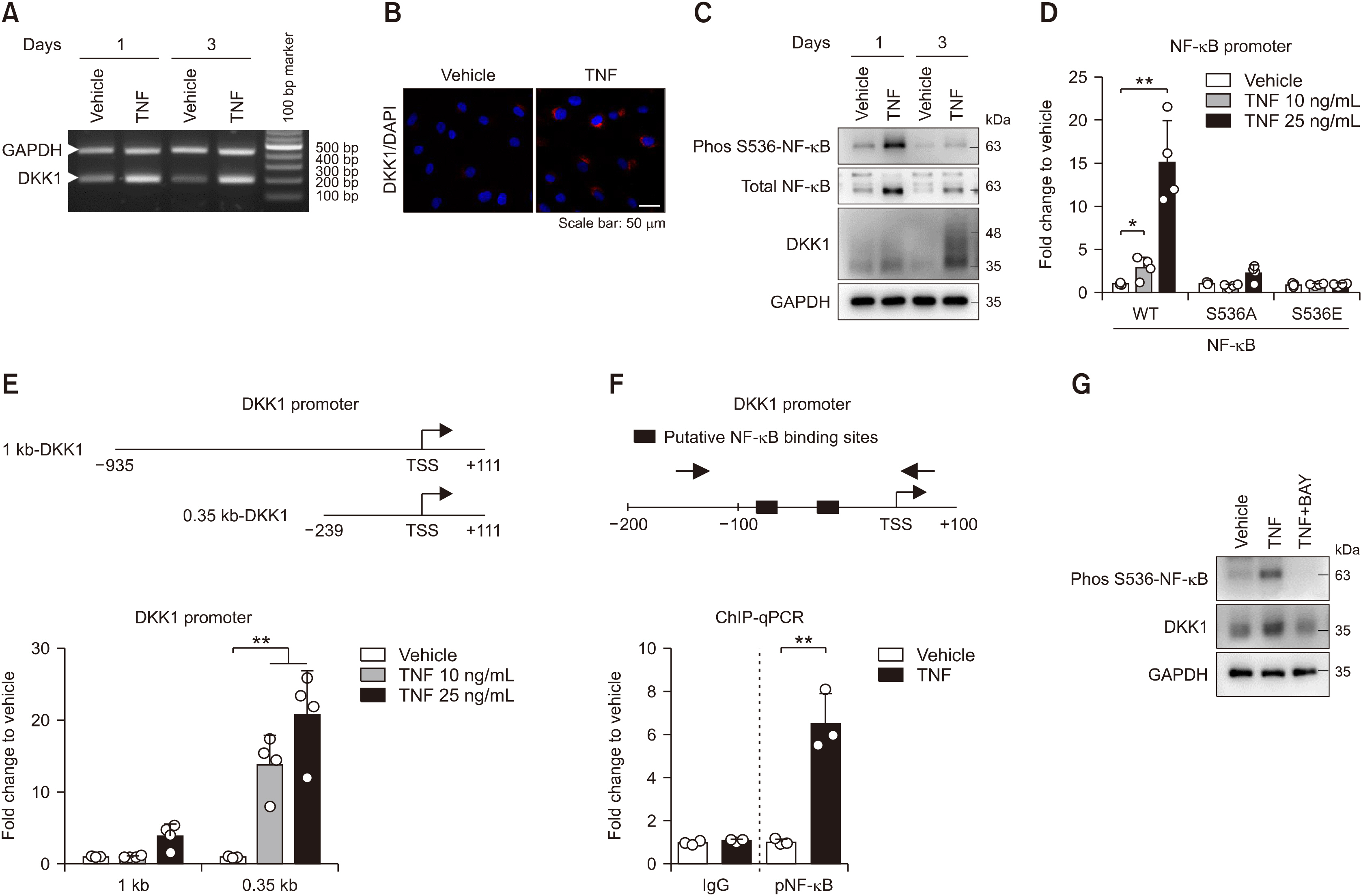
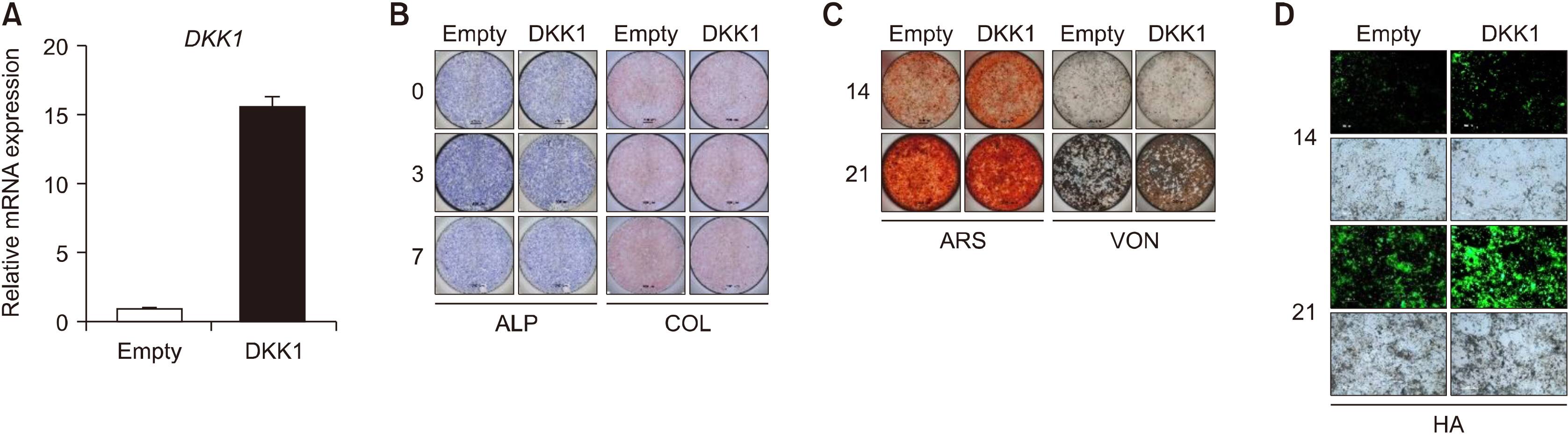
AS patients showed higher serum DKK1 levels than HCs after adjusting for age (917.4 [615.3∼1,310.0] pg/mL vs. 826.2 [670.3∼927.8] pg/mL, p=0.043). TNF treatment promoted bone formation and DKK1 expression in both control enthesis cells and those of AS. This enhanced bone formation by TNF was pronounced in AS-enthesis than those of controls. Mechanically, TNF induced NF-κB activation upregulates the DKK1 transcript level. While, NF-κB inhibitor led to downregulate DKK1 expression in the enthesis. Besides, DKK1 overexpression promoted bone formation in enthesis.
Conclusion
TNF induced DKK1 expression in the enthesis through NF-κB activation. TNF-induced DKK1 expression may play a bone formation in the radiologic progression of ankylosing spondylitis.
Figure
Cited by 1 articles
-
The Long, Dynamic Journey to the Elucidation of the Links Between Inflammation, Ectopic Bone Formation, and Wnt Signaling in Ankylosing Spondylitis
Seong-Ryul Kwon
J Rheum Dis. 2022;29(1):1-3. doi: 10.4078/jrd.2022.29.1.1.
Reference
-
1. Machado P, Landewé R, Braun J, Hermann KG, Baker D, van der Heijde D. 2010; Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis. 69:1465–70. DOI: 10.1136/ard.2009.124206. PMID: 20498215.
Article2. Kiltz U, Braun J. 2020; Assessments of functioning in patients with axial spondyloarthritis. J Rheum Dis. 27:22–9. DOI: 10.4078/jrd.2020.27.1.22.
Article3. Schett G, Lories RJ, D'Agostino MA, Elewaut D, Kirkham B, Soriano ER, et al. 2017; Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol. 13:731–41. DOI: 10.1038/nrrheum.2017.188. PMID: 29158573.
Article4. Gratacós J, Collado A, Filella X, Sanmartí R, Cañete J, Llena J, et al. 1994; Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br J Rheumatol. 33:927–31. DOI: 10.1093/rheumatology/33.10.927. PMID: 7921752.5. Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, et al. 1995; Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 38:499–505. DOI: 10.1002/art.1780380407. PMID: 7718003.
Article6. Koo BS, Oh JS, Park SY, Shin JH, Ahn GY, Lee S, et al. 2020; Tumour necrosis factor inhibitors slow radiographic progression in patients with ankylosing spondylitis: 18-year real-world evidence. Ann Rheum Dis. 79:1327–32. DOI: 10.1136/annrheumdis-2019-216741. PMID: 32660979.
Article7. Maeda K, Kobayashi Y, Koide M, Uehara S, Okamoto M, Ishihara A, et al. 2019; The regulation of bone metabolism and disorders by Wnt signaling. Int J Mol Sci. 20:5525. DOI: 10.3390/ijms20225525. PMID: 31698687. PMCID: PMC6888566.
Article8. Uderhardt S, Diarra D, Katzenbeisser J, David JP, Zwerina J, Richards W, et al. 2010; Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum Dis. 69:592–7. DOI: 10.1136/ard.2008.102046. PMID: 19304568.
Article9. Liu Q, Hu CH, Zhou CH, Cui XX, Yang K, Deng C, et al. 2015; DKK1 rescues osteogenic differentiation of mesenchymal stem cells isolated from periodontal ligaments of patients with diabetes mellitus induced periodontitis. Sci Rep. 5:13142. DOI: 10.1038/srep13142. PMID: 26278788. PMCID: PMC4538385.
Article10. Ustun N, Tok F, Kalyoncu U, Motor S, Yuksel R, Yagiz AE, et al. 2014; Sclerostin and Dkk-1 in patients with ankylosing spondylitis. Acta Reumatol Port. 39:146–51. PMID: 25111416.11. Liao HT, Lin YF, Tsai CY, Chou TC. 2018; Bone morphogenetic proteins and Dickkopf-1 in ankylosing spondylitis. Scand J Rheumatol. 47:56–61. DOI: 10.1080/03009742.2017.1287305. PMID: 28303752.
Article12. Zhang L, Ouyang H, Xie Z, Liang ZH, Wu XW. 2016; Serum DKK-1 level in the development of ankylosing spondylitis and rheumatic arthritis: a meta-analysis. Exp Mol Med. 48:e228. DOI: 10.1038/emm.2016.12. PMID: 27103566. PMCID: PMC4855274.
Article13. Wu M, Chen M, Ma Y, Yang J, Han R, Yuan Y, et al. 2018; Dickkopf-1 in ankylosing spondylitis: review and meta-analysis. Clin Chim Acta. 481:177–83. DOI: 10.1016/j.cca.2018.03.010. PMID: 29544750.
Article14. van der Linden S, Valkenburg HA, Cats A. 1984; Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27:361–8. DOI: 10.1002/art.1780270401. PMID: 6231933.15. Jo S, Won EJ, Kim MJ, Lee YJ, Jin SH, Park PR, et al. 2020; Nov. 25. STAT3 phosphorylation inhibition for treating inflammation and new bone formation in ankylosing spondylitis. Rheumatology (Oxford). [Epub]. DOI:10.1093/rheumatology/keaa846. DOI: 10.1093/rheumatology/keaa846. PMID: 33237331.
Article16. Kim HY, Park JH, Won HY, Lee JY, Kong G. 2015; CBX7 inhibits breast tumorigenicity through DKK-1-mediated suppression of the Wnt/β-catenin pathway. FASEB J. 29:300–13. DOI: 10.1096/fj.14-253997. PMID: 25351982.
Article17. Kim H, Chung H, Kim HJ, Lee JY, Oh MY, Kim Y, et al. 2008; Id-1 regulates Bcl-2 and Bax expression through p53 and NF-kappaB in MCF-7 breast cancer cells. Breast Cancer Res Treat. 112:287–96. DOI: 10.1007/s10549-007-9871-6. PMID: 18158619.18. Jo S, Wang SE, Lee YL, Kang S, Lee B, Han J, et al. 2018; IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther. 20:115. DOI: 10.1186/s13075-018-1582-3. PMID: 29880011. PMCID: PMC5992730.
Article19. Lacazette E. 2017; A laboratory practical illustrating the use of the ChIP-qPCR method in a robust model: estrogen receptor alpha immunoprecipitation using Mcf-7 culture cells. Biochem Mol Biol Educ. 45:152–60. DOI: 10.1002/bmb.20999. PMID: 27666748.
Article20. Wang SE, Ko SY, Jo S, Choi M, Lee SH, Jo HR, et al. 2017; TRPV1 regulates stress responses through HDAC2. Cell Rep. 19:401–12. DOI: 10.1016/j.celrep.2017.03.050. PMID: 28402861.
Article21. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. 2007; Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 13:156–63. DOI: 10.1038/nm1538. PMID: 17237793.
Article22. Choe JY, Kim JH, Park KY, Choi CH, Kim SK. 2016; Activation of dickkopf-1 and focal adhesion kinase pathway by tumour necrosis factor α induces enhanced migration of fibroblast-like synoviocytes in rheumatoid arthritis. Rheumatology (Oxford). 55:928–38. DOI: 10.1093/rheumatology/kev422. PMID: 26715774.
Article23. Lavocat F, Osta B, Miossec P. 2016; Increased sensitivity of rheumatoid synoviocytes to Schnurri-3 expression in TNF-α and IL-17A induced osteoblastic differentiation. Bone. 87:89–96. DOI: 10.1016/j.bone.2016.04.008. PMID: 27072520.
Article24. Osta B, Lavocat F, Eljaafari A, Miossec P. 2014; Effects of interleukin-17A on osteogenic differentiation of isolated human mesenchymal stem cells. Front Immunol. 5:425. DOI: 10.3389/fimmu.2014.00425. PMID: 25228904. PMCID: PMC4151036.
Article25. Li X, Wang J, Zhan Z, Li S, Zheng Z, Wang T, et al. 2018; Inflammation intensity-dependent expression of osteoinductive Wnt proteins is critical for ectopic new bone formation in ankylosing spondylitis. Arthritis Rheumatol. 70:1056–70. DOI: 10.1002/art.40468. PMID: 29481736.
Article26. Jo S, Yoon S, Lee SY, Kim SY, Park H, Han J, et al. 2020; DKK1 induced by 1,25D3 is required for the mineralization of osteoblasts. Cells. 9:236. DOI: 10.3390/cells9010236. PMID: 31963554. PMCID: PMC7017072.
Article27. Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, et al. 2005; Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 37:945–52. DOI: 10.1038/ng1614. PMID: 16056226.
Article28. Nam B, Park H, Lee YL, Oh Y, Park J, Kim SY, et al. 2020; TGFβ1 suppressed matrix mineralization of osteoblasts differentiation by regulating SMURF1-C/EBPβ-DKK1 axis. Int J Mol Sci. 21:9771. DOI: 10.3390/ijms21249771. PMID: 33371439. PMCID: PMC7767413.
Article29. Tuylu T, Sari I, Solmaz D, Kozaci DL, Akar S, Gunay N, et al. 2014; Fetuin-A is related to syndesmophytes in patients with ankylosing spondylitis: a case control study. Clinics (Sao Paulo). 69:688–93. DOI: 10.6061/clinics/2014(10)07. PMID: 25518021. PMCID: PMC4221327.
Article30. Huang J, Song G, Yin Z, Fu Z, Ye Z. 2016; Alteration of bone turnover markers in canonical wingless pathway in patients with ankylosing spondylitis. Arch Rheumatol. 31:221–8. DOI: 10.5606/ArchRheumatol.2016.5857. PMID: 29900942. PMCID: PMC5827846.
Article31. Daoussis D, Liossis SN, Solomou EE, Tsanaktsi A, Bounia K, Karampetsou M, et al. 2010; Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 62:150–8. DOI: 10.1002/art.27231. PMID: 20039407.
Article32. Rossini M, Viapiana O, Idolazzi L, Ghellere F, Fracassi E, Troplini S, et al. 2016; Higher level of Dickkopf-1 is associated with low bone mineral density and higher prevalence of vertebral fractures in patients with ankylosing spondylitis. Calcif Tissue Int. 98:438–45. DOI: 10.1007/s00223-015-0093-3. PMID: 26645432.
Article33. Kwon SR, Lim MJ, Suh CH, Park SG, Hong YS, Yoon BY, et al. 2012; Dickkopf-1 level is lower in patients with ankylosing spondylitis than in healthy people and is not influenced by anti-tumor necrosis factor therapy. Rheumatol Int. 32:2523–7. DOI: 10.1007/s00296-011-1981-0. PMID: 21833531.
Article34. Korkosz M, Gąsowski J, Leszczyński P, Pawlak-Buś K, Jeka S, Kucharska E, et al. 2013; High disease activity in ankylosing spondylitis is associated with increased serum sclerostin level and decreased wingless protein-3a signaling but is not linked with greater structural damage. BMC Musculoskelet Disord. 14:99. DOI: 10.1186/1471-2474-14-99. PMID: 23509994. PMCID: PMC3639156.
Article35. Sakellariou GT, Iliopoulos A, Konsta M, Kenanidis E, Potoupnis M, Tsiridis E, et al. 2017; Serum levels of Dkk-1, sclerostin and VEGF in patients with ankylosing spondylitis and their association with smoking, and clinical, inflammatory and radiographic parameters. Joint Bone Spine. 84:309–15. DOI: 10.1016/j.jbspin.2016.05.008. PMID: 27369645.
Article36. Niu CC, Lin SS, Yuan LJ, Chen LH, Yang CY, Chung AN, et al. 2017; Correlation of blood bone turnover biomarkers and Wnt signaling antagonists with AS, DISH, OPLL, and OYL. BMC Musculoskelet Disord. 18:61. DOI: 10.1186/s12891-017-1425-4. PMID: 28153008. PMCID: PMC5290649.
Article37. Li S, Yin Y, Yao L, Lin Z, Sun S, Zhang J, et al. 2020; TNF‑α treatment increases DKK1 protein levels in primary osteoblasts via upregulation of DKK1 mRNA levels and downregulation of miR‑335‑5p. Mol Med Rep. 22:1017–25. DOI: 10.3892/mmr.2020.11152. PMID: 32468044. PMCID: PMC7339467.
Article