J Korean Med Sci.
2021 Sep;36(36):e230. 10.3346/jkms.2021.36.e230.
Treatment Patterns of Type 2 Diabetes Assessed Using a Common Data Model Based on Electronic Health Records of 2000–2019
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Research Institute of Clinical Medicine of Jeonbuk National University-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonbuk National University Medical School, Jeonju, Korea
- 2Center for Clinical Pharmacology, Biochemical Research Institute, Jeonbuk National University Hospital, Jeonju, Korea
- KMID: 2520109
- DOI: http://doi.org/10.3346/jkms.2021.36.e230
Abstract
- Background
Real-world data analysis is useful for identifying treatment patterns. Understanding drug prescription patterns of type 2 diabetes mellitus may facilitate diabetes management. We aimed to analyze treatment patterns of type 2 diabetes mellitus using Observational Medical Outcomes Partnership Common Data Model based on electronic health records.
Methods
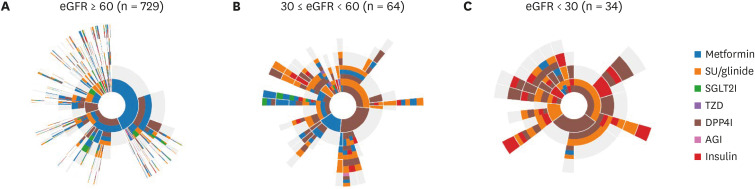
This retrospective, observational study employed electronic health records of patients who visited Jeonbuk National University Hospital in Korea during January 2000– December 2019. Data were transformed into the Observational Medical Outcomes Partnership Common Data Model and analyzed using R version 4.0.3 and ATLAS ver. 2.7.6. Prescription frequency for each anti-diabetic drug, combination therapy pattern, and prescription pattern according to age, renal function, and glycated hemoglobin were analyzed.
Results
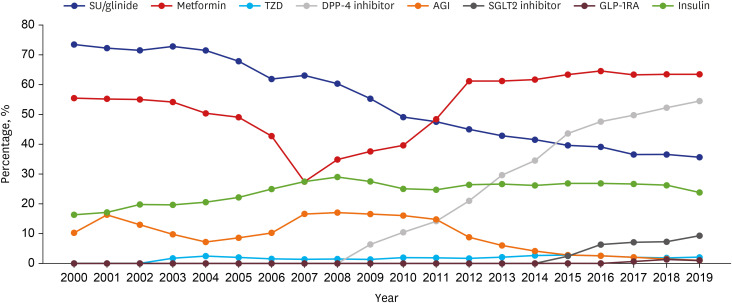
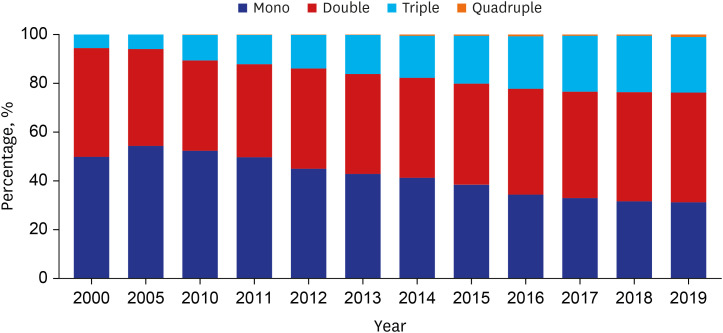
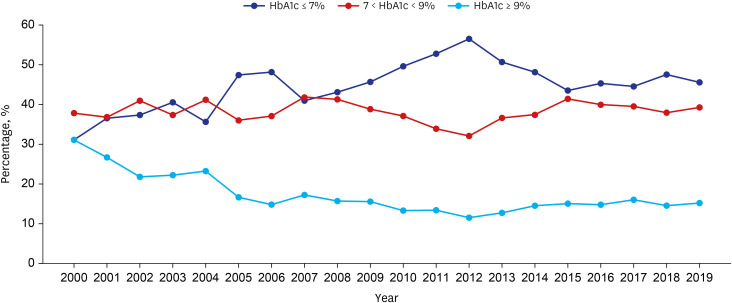
The number of adults treated for type 2 diabetes mellitus increased from 1,867 (2.0%) in 2000 to 9,972 (5.9%) in 2019. In the early 2000s, sulfonylurea was most commonly prescribed (73%), and in the recent years, metformin has been most commonly prescribed (64%). Prescription rates for DPP4 and SGLT2 inhibitors have increased gradually over the past few years. Monotherapy prescription rates decreased, whereas triple and quadruple combination prescription rates increased steadily. Different drug prescription patterns according to age, renal function, and glycated hemoglobin were observed. The proportion of patients with HbA1c ≤ 7% increased from 31.1% in 2000 to 45.6% in 2019, but that of patients visiting the emergency room for severe hypoglycemia did not change over time.
Conclusion
Medication utilization patterns have changed significantly over the past 20 years with an increase in the use of newer drugs and a shift to combination therapies. In addition, various prescription patterns were demonstrated according to the patient characteristics in actual practice. Although glycemic control has improved, the proportion within the target is still low, underscoring the need to improve diabetes management.
Figure
Cited by 2 articles
-
Advanced Liver Fibrosis Is Associated with Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease (
Diabetes Metab J 2022;46:630-9)
Da Hea Seo, So Hun Kim
Diabetes Metab J. 2022;46(6):956-957. doi: 10.4093/dmj.2022.0381.Real-World Treatment Intensity and Patterns in Patients With Myopic Choroidal Neovascularization: Common Data Model in Ophthalmology
Manh-Hung Bui, Da Yun Lee, Sang Jun Park, Kyu Hyung Park
J Korean Med Sci. 2023;38(23):e174. doi: 10.3346/jkms.2023.38.e174.
Reference
-
1. Jung CH, Son JW, Kang S, Kim WJ, Kim HS, Kim HS, et al. Diabetes fact sheets in Korea, 2020: an appraisal of current status. Diabetes Metab J. 2021; 45(1):1–10. PMID: 33434426.
Article2. Kim BY, Won JC, Lee JH, Kim HS, Park JH, Ha KH, et al. Diabetes fact sheets in Korea, 2018: an appraisal of current status. Diabetes Metab J. 2019; 43(4):487–494. PMID: 31339012.
Article3. White JR Jr. A brief history of the development of diabetes medications. Diabetes Spectr. 2014; 27(2):82–86. PMID: 26246763.
Article4. Giugliano D, Longo M, Maiorino MI, Bellastella G, Chiodini P, Solerte SB, et al. Efficacy of SGLT-2 inhibitors in older adults with diabetes: systematic review with meta-analysis of cardiovascular outcome trials. Diabetes Res Clin Pract. 2020; 162:108114. PMID: 32165164.
Article5. Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019; 7(10):776–785. PMID: 31422062.
Article6. Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, et al. 2019 clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019; 43(4):398–406. PMID: 31441247.
Article7. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2021. Diabetes Care. 2021; 44(Suppl 1):S111–S124. PMID: 33298420.8. Bang C, Mortensen MB, Lauridsen KG, Bruun JM. Trends in antidiabetic drug utilization and expenditure in Denmark: a 22-year nationwide study. Diabetes Obes Metab. 2020; 22(2):167–172. PMID: 31486269.
Article9. Ko SH, Kim DJ, Park JH, Park CY, Jung CH, Kwon HS, et al. Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002–2013: nationwide population-based cohort study. Medicine (Baltimore). 2016; 95(27):e4018. PMID: 27399082.10. Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015; 216:574–578. PMID: 26262116.11. Jeon Y, Choi Y, Kim EH, Oh S, Lee H. Common data model-based real-world data for practical clinical practice guidelines: clinical pharmacology perspectives. Transl Clin Pharmacol. 2020; 28(2):67–72. PMID: 32656157.
Article12. Hripcsak G, Ryan PB, Duke JD, Shah NH, Park RW, Huser V, et al. Characterizing treatment pathways at scale using the OHDSI network. Proc Natl Acad Sci U S A. 2016; 113(27):7329–7336. PMID: 27274072.
Article13. Kim H, Yoo S, Jeon Y, Yi S, Kim S, Choi SA, et al. Characterization of anti-seizure medication treatment pathways in pediatric epilepsy using the electronic health record-based common data model. Front Neurol. 2020; 11:409. PMID: 32477256.
Article14. Zhang X, Wang L, Miao S, Xu H, Yin Y, Zhu Y, et al. Analysis of treatment pathways for three chronic diseases using OMOP CDM. J Med Syst. 2018; 42(12):260. PMID: 30421323.
Article15. Yoon D, Ahn EK, Park MY, Cho SY, Ryan P, Schuemie MJ, et al. Conversion and data quality assessment of electronic health record data at a Korean tertiary teaching hospital to a common data model for distributed network research. Healthc Inform Res. 2016; 22(1):54–58. PMID: 26893951.
Article16. Won JC, Lee JH, Kim JH, Kang ES, Won KC, Kim DJ, et al. Diabetes fact sheet in Korea, 2016: an appraisal of current status. Diabetes Metab J. 2018; 42(5):415–424. PMID: 30113146.
Article17. Cahn A, Cefalu WT. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care. 2016; 39(Suppl 2):S137–S145. PMID: 27440826.
Article18. Korean Diabetes Association. Diabetes fact sheet in Korea 2018. Updated 2020. Accessed May 12, 2021. https://www.diabetes.or.kr/pro/news/admin.php?category=A&code=admin&mode=view&number=1615.19. Karter AJ, Subramanian U, Saha C, Crosson JC, Parker MM, Swain BE, et al. Barriers to insulin initiation: the translating research into action for diabetes insulin starts project. Diabetes Care. 2010; 33(4):733–735. PMID: 20086256.
Article20. Cho YK, Lee J, Kim HS, Park JY, Jung CH, Lee WJ. Clinical efficacy of quadruple oral therapy for type 2 diabetes in real-world practice: a retrospective observational study. Diabetes Ther. 2020; 11(9):2029–2039. PMID: 32696268.21. Moon JS, Suh S, Kim SS, Jin HY, Kim JM, Jang MH, et al. Efficacy and safety of treatment with quadruple oral hypoglycemic agents in uncontrolled type 2 diabetes mellitus: a multi-center, retrospective, observational study. Diabetes Metab J. Forthcoming. 2020; DOI: 10.4093/dmj.2020.0107.
Article22. Grant RW, Wexler DJ, Watson AJ, Lester WT, Cagliero E, Campbell EG, et al. How doctors choose medications to treat type 2 diabetes: a national survey of specialists and academic generalists. Diabetes Care. 2007; 30(6):1448–1453. PMID: 17337497.
Article23. Ko SH, Kim SR, Kim DJ, Oh SJ, Lee HJ, Shim KH, et al. 2011 clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J. 2011; 35(5):431–436. PMID: 22111032.
Article24. Paolisso G, Monami M, Marfella R, Rizzo MR, Mannucci E. Dipeptidyl peptidase-4 inhibitors in the elderly: more benefits or risks? Adv Ther. 2012; 29(3):218–233. PMID: 22411425.
Article25. Nelinson DS, Sosa JM, Chilton RJ. SGLT2 inhibitors: a narrative review of efficacy and safety. J Osteopath Med. 2021; 121(2):229–239. PMID: 33567084.
Article26. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998; 352(9131):854–865. PMID: 9742977.27. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. Updated 2017. Accessed May 12, 2021. http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medicine in the Fourth Industrial Revolution: What Should We Prepare?
- Technological Innovations Transforming Diabetes Care in Practice
- A Comparison of the Nursing Records of Hysterectomy Patients: Pre and Post Implementation of an ICNP Based Electronic Nursing Record System
- Comparison of Nursing Records of Open Heart Surgery Patients before and after Implementation of Electronic Nursing Record
- Precision Medicine in Type 2 Diabetes