Ann Surg Treat Res.
2021 Sep;101(3):160-166. 10.4174/astr.2021.101.3.160.
Adjuvant oxaliplatin-based chemotherapy effect after treatment of colorectal hepatic metastasis
- Affiliations
-
- 1Department of Surgery, Colorectal Cancer Center, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
- KMID: 2519846
- DOI: http://doi.org/10.4174/astr.2021.101.3.160
Abstract
- Purpose
We aimed to investigate whether adjuvant oxaliplatin-based chemotherapy after treatment for hepatic metastasis affects recurrence or survival and to determine the risk factors for recurrence or survival.
Methods
Forty-six patients who underwent curative treatment for hepatic metastasis from colorectal cancer between July 2009 and December 2017 were included from a retrospectively collected patient database. Curative resection included hepatic resection, radiofrequency ablation (RFA), or a combination of both, followed by adjuvant chemotherapy with oxaliplatin-based chemotherapy.
Results
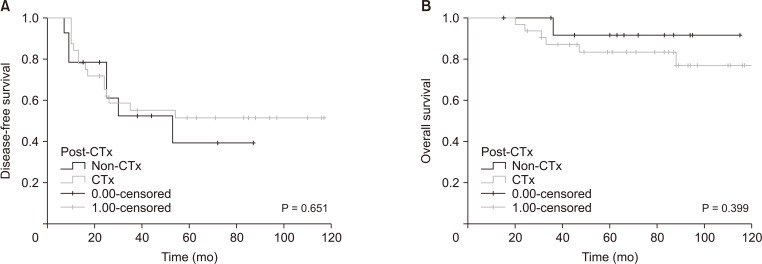
Thirty-seven patients (80.4%) had colon cancer and 9 (19.6%) had rectal cancer. Twenty-six patients (56.5%) underwent hepatic resection, 7 (15.2%) RFA, and 13 (28.3%) hepatic resection and RFA. Thirty-two patients (69.6%) underwent chemotherapy after hepatic treatment. The recurrence incidence was 50% in the non-chemotherapy group and 46.9% in the chemotherapy group (P > 0.999). The incidence of death was 7.1% in the non-chemotherapy group and 18.8% in the chemotherapy group (P = 0.657). The recurrence risk factors were N stage (N0 vs. N2; P = 0.013, P = 0.005) and bilobed hepatic metastasis (P = 0.027, P = 0.009) in the univariate and multivariate analyses, respectively. However, chemotherapy after hepatic treatment was not a risk factor for disease-free survival (DFS) or overall survival (OS) in the univariate and multivariate analyses (P = 0.656 and P = 0.414, respectively; P = 0.510 and P = 0.459, respectively).
Conclusion
Oxaliplatin-based adjuvant chemotherapy after colorectal hepatic metastasis treatment did not affect the DFS or OS. The N stage of the primary tumor and bilobed hepatic metastasis are risk factors for recurrence and death.
Keyword
Figure
Cited by 1 articles
-
National cancer screening program for colorectal cancer in Korea
Seung Min Baik, Ryung-Ah Lee
Ann Surg Treat Res. 2023;105(6):333-340. doi: 10.4174/astr.2023.105.6.333.
Reference
-
1. Dekker E, Tanis PJ, Vleugels JL, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019; 394:1467–1480. PMID: 31631858.
Article2. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases: a population-based study on incidence, management and survival. BMC Cancer. 2018; 18:78. PMID: 29334918.
Article3. van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015; 32:457–465. PMID: 25899064.
Article4. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014; 14:810. PMID: 25369977.
Article5. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006; 244:254–259. PMID: 16858188.
Article6. Angelsen JH, Viste A, Løes IM, Eide GE, Hoem D, Sorbye H, et al. Predictive factors for time to recurrence, treatment and post-recurrence survival in patients with init ial ly resected colorectal liver metastases. World J Surg Oncol. 2015; 13:328. PMID: 26631156.
Article7. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013; 14:1208–1215. PMID: 24120480.
Article8. Kanemitsu Y, Kato T, Shimizu Y, Inaba Y, Shimada Y, Nakamura K, et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol. 2009; 39:406–409. PMID: 19389795.
Article9. Kemeny MM, Adak S, Gray B, Macdonald JS, Smith T, Lipsitz S, et al. Combined-modalit y treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy: an intergroup study. J Clin Oncol. 2002; 20:1499–1505. PMID: 11896097.10. Parks R, Gonen M, Kemeny N, Jarnagin W, D'Angelica M, DeMatteo R, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007; 204:753–763. PMID: 17481478.
Article11. Petrelli NJ. Perioperative or adjuvant therapy for resectable colorectal hepatic metastases. J Clin Oncol. 2008; 26:4862–4863. PMID: 18794535.
Article12. Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006; 24:4976–4982. PMID: 17075115.
Article13. Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999; 341:2039–2048. PMID: 10615075.
Article14. Kemeny N, Capanu M, D'Angelica M, Jarnagin W, Haviland D, Dematteo R, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Ann Oncol. 2009; 20:1236–1241. PMID: 19233901.
Article15. Chaudhury P, Hassanain M, Bouganim N, Salman A, Kavan P, Metrakos P. Perioperat ive chemotherapy with bevacizumab and liver resection for colorectal cancer liver metastasis. HPB (Oxford). 2010; 12:37–42. PMID: 20495643.16. Bridgewater JA, Pugh SA, Maishman T, Eminton Z, Mellor J, Whitehead A, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020; 21:398–411. PMID: 32014119.17. Mima K, Beppu T, Chikamoto A, Miyamoto Y, Nakagawa S, Kuroki H, et al. Hepatic resection combined with radiofrequency ablation for initially unresectable colorectal liver metastases after effective chemotherapy is a safe procedure with a low incidence of local recurrence. Int J Clin Oncol. 2013; 18:847–855. PMID: 22940848.
Article18. Kim YI, Park IJ, Kim JE, Kim SY, Park JH, Lee JH, et al. Hepatic resection after neoadjuvant chemotherapy for patients with liver metastases from colorectal cancer: need for cautious planning. Ann Surg Treat Res. 2019; 97:245–253. PMID: 31742209.
Article19. Neo EL, Beeke C, Price T, Maddern G, Karapetis C, Luke C, et al. South Australian clinical registry for metastatic colorectal cancer. ANZ J Surg. 2011; 81:352–357. PMID: 21518185.
Article20. van der Pool AE, Lalmahomed ZS, Ozbay Y, de Wilt JH, Eggermont AM, Jzermans JN, et al. ‘Staged’ liver resection in synchronous and metachronous colorectal hepatic metastases: differences in clinicopathological features and outcome. Colorectal Dis. 2010; 12(10 Online):e229–e235. PMID: 19912286.
Article21. Ng WW, Cheung YS, Wong J, Lee KF, Lai PB. A preliminary analysis of combined liver resection with new chemotherapy for synchronous and metachronous colorectal liver metastasis. Asian J Surg. 2009; 32:189–197. PMID: 19892621.
Article