J Korean Assoc Oral Maxillofac Surg.
2021 Aug;47(4):269-278. 10.5125/jkaoms.2021.47.4.269.
Comparison of immunohistochemical analysis on sinus augmentation using demineralized tooth graft and bovine bone
- Affiliations
-
- 1Department of Dentistry and Oral and Maxillofacial Surgery, School of Medicine, Daegu Catholic University, Korea
- 2Department of Oral Medicine, School of Dentistry, Kyungpook National University, Korea
- 3Department of Dentistry and Prosthodontics, School of Medicine, Daegu Catholic University, Korea
- 4Department of Anatomy, School of Medicine, Daegu Catholic University, Daegu, Korea
- KMID: 2519824
- DOI: http://doi.org/10.5125/jkaoms.2021.47.4.269
Abstract
Objectives
The purpose of this animal research was to compare bone regeneration in augmented rabbit maxillary sinuses treated with demineralized particulate human-tooth graft and anorganic bovine bone by immunohistochemical analysis.
Materials and Methods
Piezoelectric bilateral sinus augmentation was performed in eight adult rabbits. In the control group, anorganic bovine was grafted in the maxillary sinus following elevation of the sinus membrane. In the experimental group, demineralized human particulate tooth bone was grafted in the sinus. Bone regeneration in augmented sinuses was evaluated by immunohistochemical analysis using various markers of osteoprogenitor cells.
Results
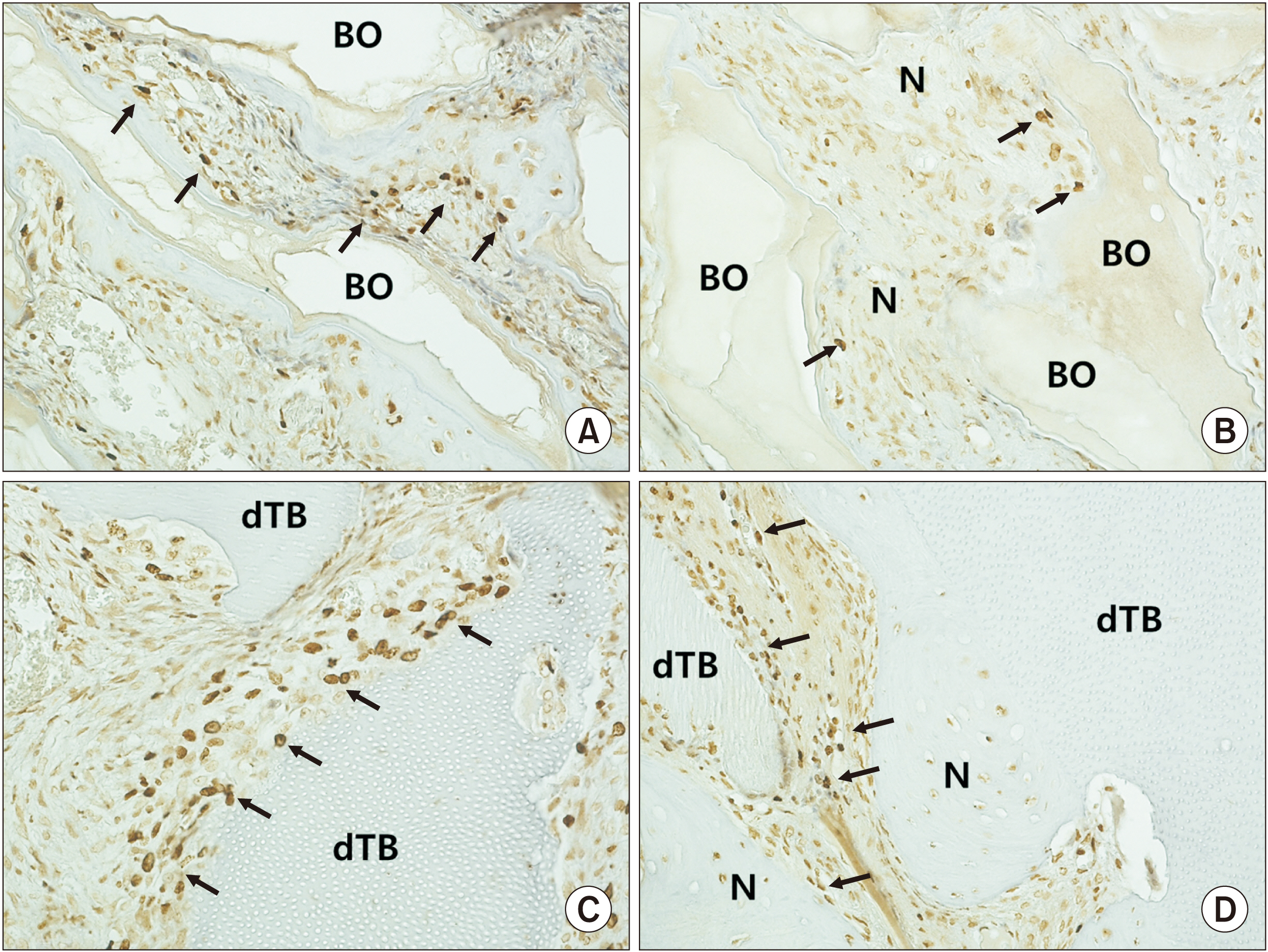
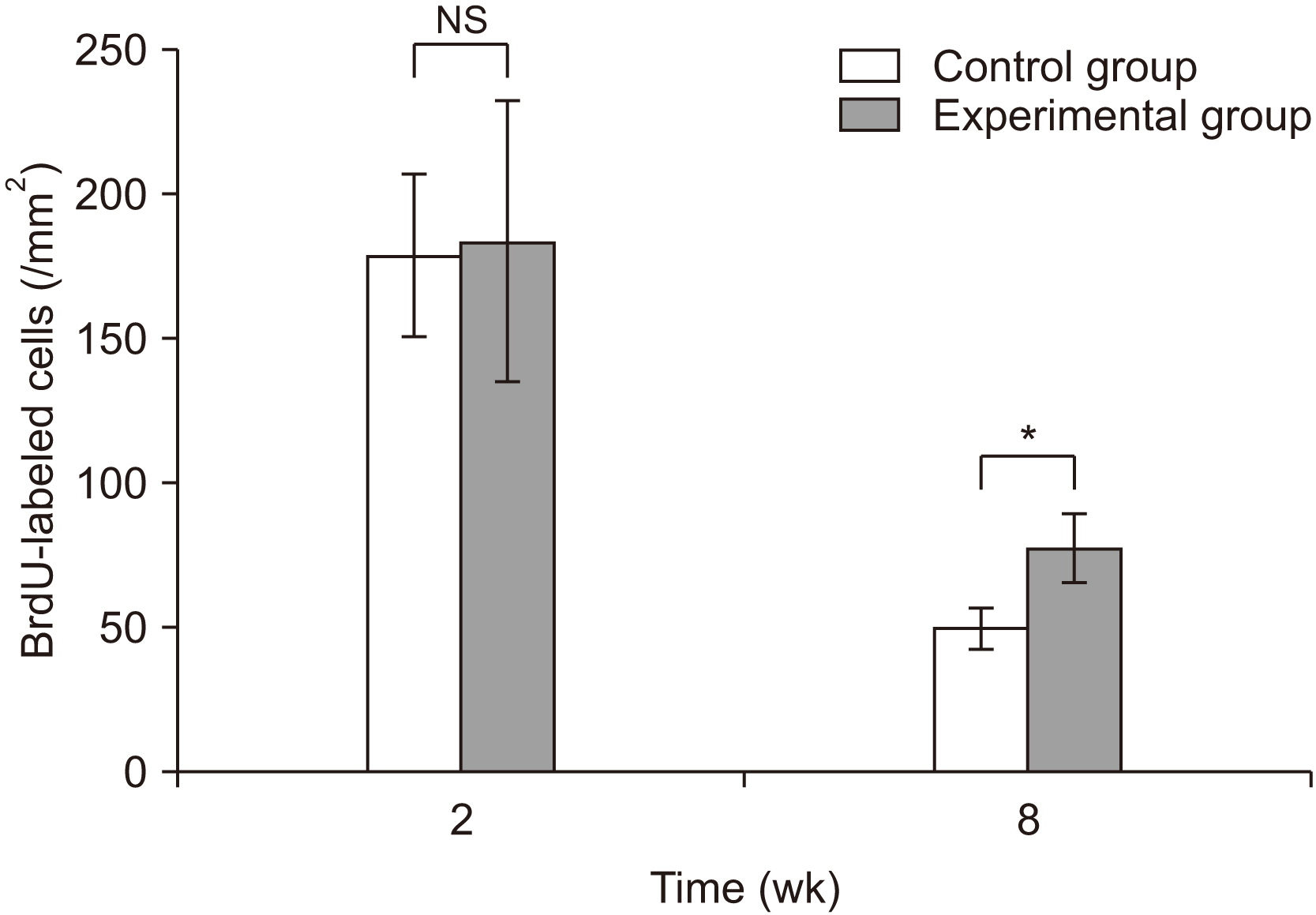
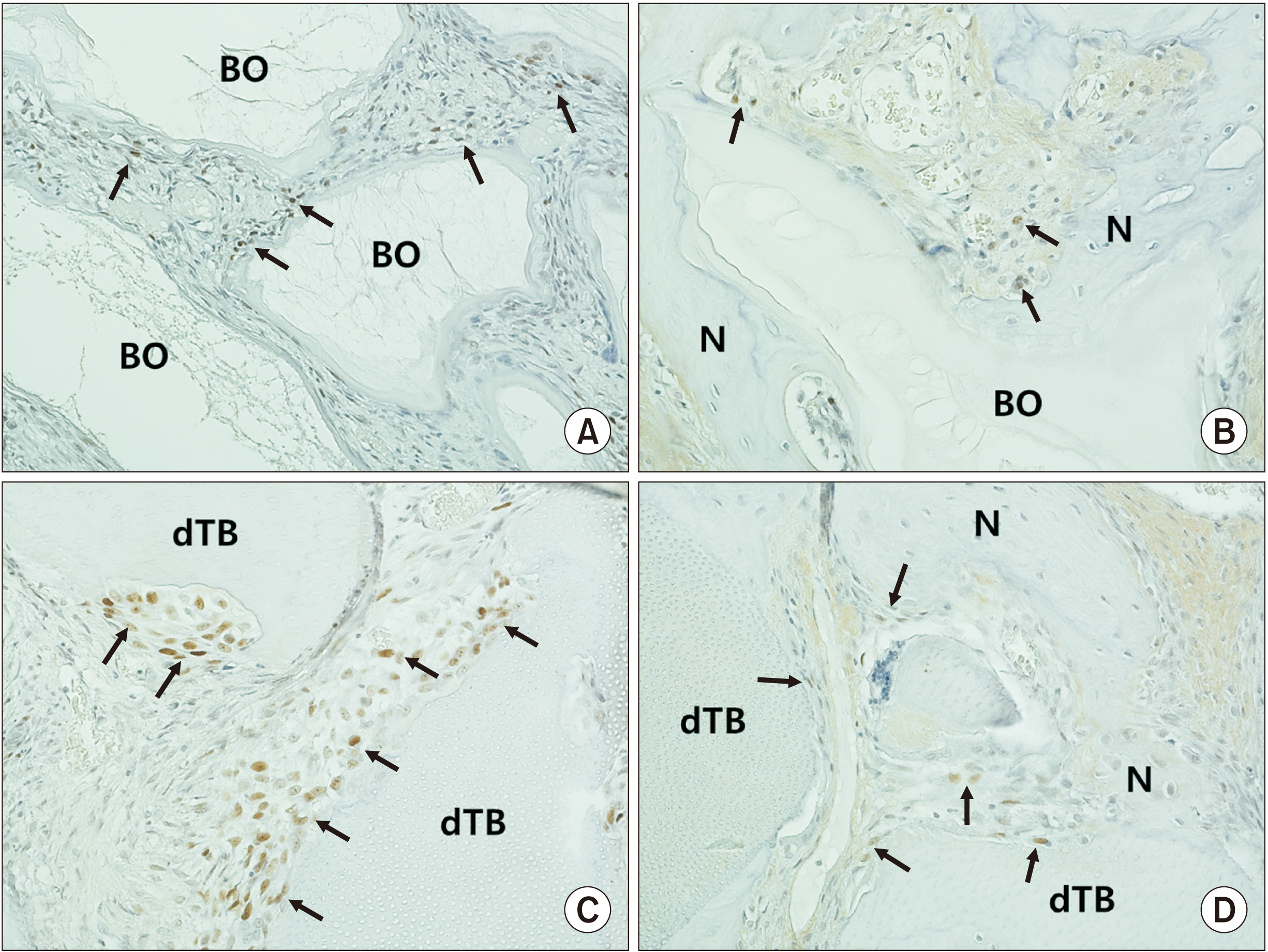
The number of bromodeoxyuridine-labeled cells was significantly higher in the experimental group than in the control group at eight weeks. The immunoreactivity of proliferating-cell nuclear antigen was increased slightly in the experimental group relative to the control group at eight weeks. Other bone markers were expressed equally in the two groups.
Conclusion
In the rabbit maxillary sinus, higher osteoinduction was correlated with demineralized human particulate tooth bone grafting than with anorganic bovine grafting.
Keyword
Figure
Reference
-
References
1. Misch CE. 1987; Maxillary sinus augmentation for endosteal implants: organized alternative treatment plans. Int J Oral Implantol. 4:49–58. PMID: 3269837.2. Aghaloo TL, Moy PK. 2007; Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 22 Suppl:49–70. PMID: 18437791.3. Duan DH, Fu JH, Qi W, Du Y, Pan J, Wang HL. 2017; Graft-free maxillary sinus floor elevation: a systematic review and meta-analysis. J Periodontol. 88:550–64. https://doi.org/10.1902/jop.2017.160665 . DOI: 10.1902/jop.2017.160665. PMID: 28168901.
Article4. Kent JN, Block MS. 1989; Simultaneous maxillary sinus floor bone grafting and placement of hydroxylapatite-coated implants. J Oral Maxillofac Surg. 47:238–42. https://doi.org/10.1016/0278-2391(89)90225-5 . DOI: 10.1016/0278-2391(89)90225-5. PMID: 2646403.
Article5. Wagner JR. 1991; A 3 1/2-year clinical evaluation of resorbable hydroxylapatite OsteoGen (HA Resorb) used for sinus lift augmentations in conjunction with the insertion of endosseous implants. J Oral Implantol. 17:152–64. PMID: 1667323.6. Cordioli G, Mazzocco C, Schepers E, Brugnolo E, Majzoub Z. 2001; Maxillary sinus floor augmentation using bioactive glass granules and autogenous bone with simultaneous implant placement. Clinical and histological findings. Clin Oral Implants Res. 12:270–8. https://doi.org/10.1034/j.1600-0501.2001.012003270.x . DOI: 10.1034/j.1600-0501.2001.012003270.x. PMID: 11359485.
Article7. Moon JW, Sohn DS, Heo JU, Kim JS. 2015; Comparison of two kinds of bovine bone in maxillary sinus augmentation: a histomorphometric study. Implant Dent. 24:19–24. https://doi.org/10.1097/ID.0000000000000187 . DOI: 10.1097/ID.0000000000000187. PMID: 25621547.
Article8. Lee KH, Kim YK, Cho WJ, Um IW, Murata M, Mitsugi M. 2015; Autogenous tooth bone graft block for sinus augmentation with simultaneous implant installation: a technical note. J Korean Assoc Oral Maxillofac Surg. 41:284–9. https://doi.org/10.5125/jkaoms.2015.41.5.284 . DOI: 10.5125/jkaoms.2015.41.5.284. PMID: 26568934. PMCID: PMC4641223.
Article9. Boyne PJ, James RA. 1980; Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 38:613–6. PMID: 6993637.10. van den Bergh JP, ten Bruggenkate CM, Krekeler G, Tuinzing DB. 1998; Sinusfloor elevation and grafting with autogenous iliac crest bone. Clin Oral Implants Res. 9:429–35. https://doi.org/10.1034/j.1600-0501.1996.090608.x . DOI: 10.1034/j.1600-0501.1996.090608.x. PMID: 11429944.
Article11. Yeomans JD, Urist MR. 1967; Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 12:999–1008. https://doi.org/10.1016/0003-9969(67)90095-7 . DOI: 10.1016/0003-9969(67)90095-7. PMID: 4226721.
Article12. Bessho K, Tagawa T, Murata M. 1990; Purification of rabbit bone morphogenetic protein derived from bone, dentin, and wound tissue after tooth extraction. J Oral Maxillofac Surg. 48:162–9. https://doi.org/10.1016/s0278-2391(10)80204-6 . DOI: 10.1016/s0278-2391(10)80204-6. PMID: 2299457.
Article13. Jun SH, Ahn JS, Lee JI, Ahn KJ, Yun PY, Kim YK. 2014; A prospective study on the effectiveness of newly developed autogenous tooth bone graft material for sinus bone graft procedure. J Adv Prosthodont. 6:528–38. https://doi.org/10.4047/jap.2014.6.6.528 . DOI: 10.4047/jap.2014.6.6.528. PMID: 25551014. PMCID: PMC4279053.
Article14. Kim YK, Lee J, Yun JY, Yun PY, Um IW. 2014; Comparison of autogenous tooth bone graft and synthetic bone graft materials used for bone resorption around implants after crestal approach sinus lifting: a retrospective study. J Periodontal Implant Sci. 44:216–21. https://doi.org/10.5051/jpis.2014.44.5.216 . DOI: 10.5051/jpis.2014.44.5.216. PMID: 25368809. PMCID: PMC4216397.
Article15. Kim ES. 2015; Autogenous fresh demineralized tooth graft prepared at chairside for dental implant. Maxillofac Plast Reconstr Surg. 37:8. https://doi.org/10.1186/s40902-015-0009-1 . DOI: 10.1186/s40902-015-0009-1. PMID: 25705613. PMCID: PMC4331600.
Article16. Kim YK, Lee JH, Um IW, Cho WJ. 2016; Guided bone regeneration using demineralized dentin matrix: long-term follow-up. J Oral Maxillofac Surg. 74:515.e1–9. https://doi.org/10.1016/j.joms.2015.10.030 . DOI: 10.1016/j.joms.2015.10.030. PMID: 26679551.
Article17. Kim ES, Kang JY, Kim JJ, Kim KW, Lee EY. 2016; Space maintenance in autogenous fresh demineralized tooth blocks with platelet-rich plasma for maxillary sinus bone formation: a prospective study. Springerplus. 5:274. https://doi.org/10.1186/s40064-016-1886-1 . DOI: 10.1186/s40064-016-1886-1. PMID: 27047706. PMCID: PMC4779789.
Article18. Lee J, Lee EY, Park EJ, Kim ES. 2015; An alternative treatment option for a bony defect from large odontoma using recycled demineralization at chairside. J Korean Assoc Oral Maxillofac Surg. 41:109–15. https://doi.org/10.5125/jkaoms.2015.41.2.109 . DOI: 10.5125/jkaoms.2015.41.2.109. PMID: 25922824. PMCID: PMC4411726.
Article19. Kabir MA, Murata M, Akazawa T, Kusano K, Yamada K, Ito M. 2017; Evaluation of perforated demineralized dentin scaffold on bone regeneration in critical-size sheep iliac defects. Clin Oral Implants Res. 28:e227–35. https://doi.org/10.1111/clr.13000 . DOI: 10.1111/clr.13000. PMID: 28097682.
Article20. Xu X, Sohn DS, Kim HG, Lee SJ, Moon YS. 2018; Comparative histomorphometric analysis of maxillary sinus augmentation with deproteinized bovine bone and demineralized particulate human tooth graft: an experimental study in rabbits. Implant Dent. 27:324–31. https://doi.org/10.1097/ID.0000000000000755 . DOI: 10.1097/ID.0000000000000755. PMID: 29613862.
Article21. Ramos-Vara JA, Miller MA. 2014; When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry--the red, brown, and blue technique. Vet Pathol. 51:42–87. https://doi.org/10.1177/0300985813505879 . DOI: 10.1177/0300985813505879. PMID: 24129895.
Article22. Dolbeare F. 1995; Bromodeoxyuridine: a diagnostic tool in biology and medicine, part I: historical perspectives, histochemical methods and cell kinetics. Histochem J. 27:339–69. PMID: 7657555.
Article23. Okafuji N, Liu ZJ, King GJ. 2006; Assessment of cell proliferation during mandibular distraction osteogenesis in the maturing rat. Am J Orthod Dentofacial Orthop. 130:612–21. https://doi.org/10.1016/j.ajodo.2005.06.023 . DOI: 10.1016/j.ajodo.2005.06.023. PMID: 17110258.
Article24. Shimada A, Shibata T, Komatsu K, Nifuji A. 2008; Improved methods for immunohistochemical detection of BrdU in hard tissue. J Immunol Methods. 339:11–6. https://doi.org/10.1016/j.jim.2008.07.013 . DOI: 10.1016/j.jim.2008.07.013. PMID: 18718840.
Article25. Prelich G, Tan CK, Kostura M, Mathews MB, So AG, Downey KM, et al. 1987; Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 326:517–20. https://doi.org/10.1038/326517a0 . DOI: 10.1038/326517a0. PMID: 2882424.
Article26. Garcia RL, Coltrera MD, Gown AM. 1989; Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 134:733–9. PMID: 2565087. PMCID: PMC1879787.27. Mandir N, Englyst H, Goodlad RA. 2008; Resistant carbohydrates stimulate cell proliferation and crypt fission in wild-type mice and in the Apc(Min/+) mouse model of intestinal cancer, association with enhanced polyp development. Br J Nutr. 100:711–21. https://doi.org/10.1017/S0007114508901276 . DOI: 10.1017/S0007114508901276. PMID: 18279550.
Article28. Yasui N, Sato M, Ochi T, Kimura T, Kawahata H, Kitamura Y, et al. 1997; Three modes of ossification during distraction osteogenesis in the rat. J Bone Joint Surg Br. 79:824–30. https://doi.org/10.1302/0301-620x.79b5.7423 . DOI: 10.1302/0301-620x.79b5.7423. PMID: 9331045.
Article29. Sodek J, Ganss B, McKee MD. 2000; Osteopontin. Crit Rev Oral Biol Med. 11:279–303. https://doi.org/10.1177/10454411000110030101 . DOI: 10.1177/10454411000110030101. PMID: 11021631.
Article30. Standal T, Borset M, Sundan A. 2004; Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 26:179–84. PMID: 15494684.31. Desbois C, Karsenty G. 1995; Osteocalcin cluster: implications for functional studies. J Cell Biochem. 57:379–83. https://doi.org/10.1002/jcb.240570302 . DOI: 10.1002/jcb.240570302. PMID: 7768973.
Article32. Sato M, Yasui N, Nakase T, Kawahata H, Sugimoto M, Hirota S, et al. 1998; Expression of bone matrix proteins mRNA during distraction osteogenesis. J Bone Miner Res. 13:1221–31. https://doi.org/10.1359/jbmr.1998.13.8.1221 . DOI: 10.1359/jbmr.1998.13.8.1221. PMID: 9718189.
Article33. Sakamoto A, Oda Y, Iwamoto Y, Tsuneyoshi M. 1999; A comparative study of fibrous dysplasia and osteofibrous dysplasia with regard to expressions of c-fos and c-jun products and bone matrix proteins: a clinicopathologic review and immunohistochemical study of c-fos, c-jun, type I collagen, osteonectin, osteopontin, and osteocalcin. Hum Pathol. 30:1418–26. https://doi.org/10.1016/s0046-8177(99)90162-4 . DOI: 10.1016/s0046-8177(99)90162-4. PMID: 10667418.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Double Layers Technique for Maxillary Sinus Augmentation with Demineralized and Mineralized Bone Graft Materials
- Maxillary sinus bone graft using particulated ramal autobone and bovine bone
- Familial tooth bone graft for ridge and sinus augmentation: a report of two cases
- Histomorphometric study of rabbit's maxillary sinus augmentation with various graft materials
- The Efficacy of the Graft Materials after Sinus Elevation: Retrospective Comparative Study Using Panoramic Radiography