Ann Hepatobiliary Pancreat Surg.
2021 Aug;25(3):440-444. 10.14701/ahbps.2021.25.3.440.
Curative resection of bladder cancer with pancreas head metastasis
- Affiliations
-
- 1Department of Surgery, Biomedical Research Institute and Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 2Department of Radiology, Biomedical Research Institute and Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 3Department of Urology, Biomedical Research Institute and Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 4Department of Pathology, Biomedical Research Institute and Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- KMID: 2519306
- DOI: http://doi.org/10.14701/ahbps.2021.25.3.440
Abstract
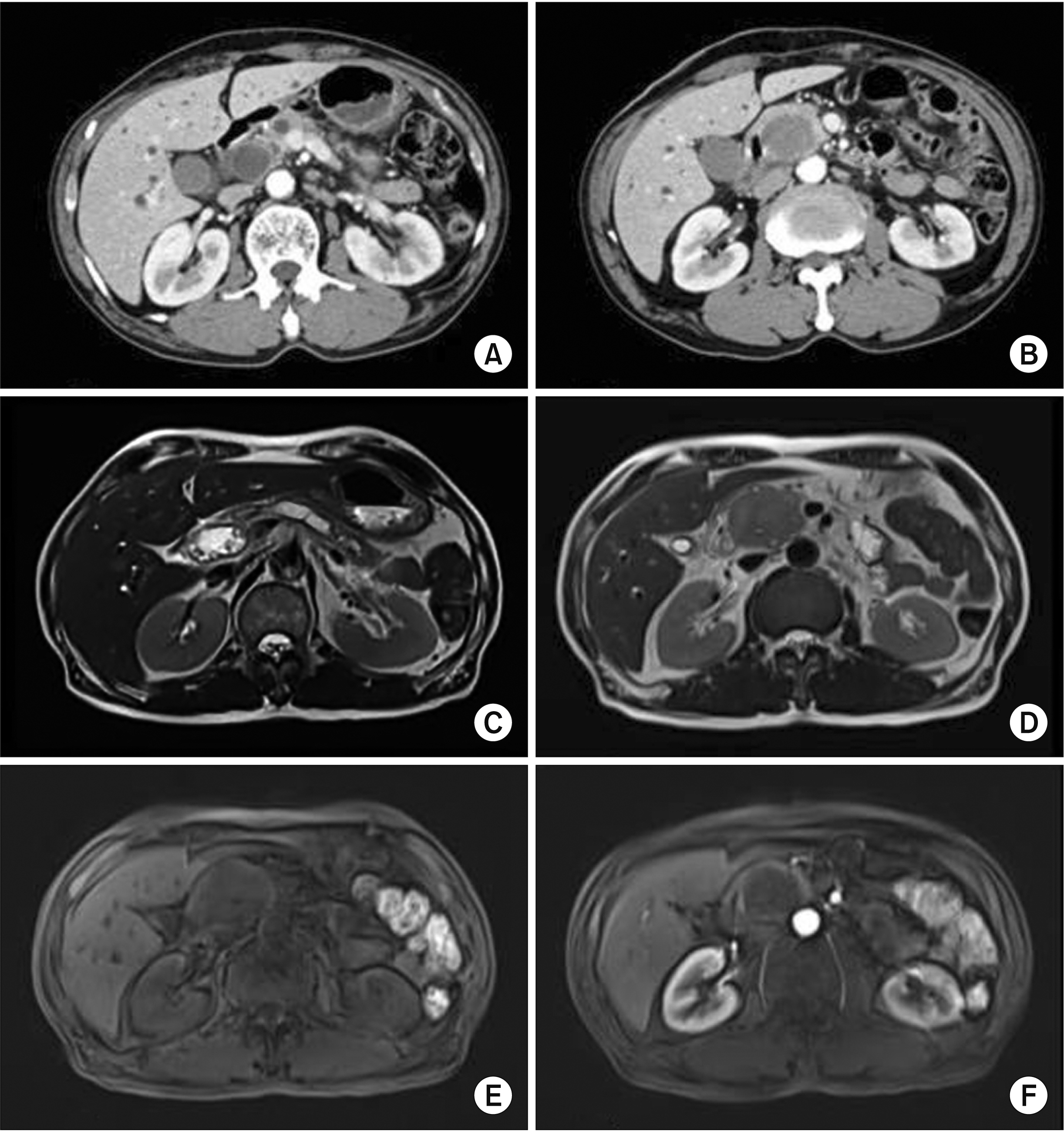
- Bladder cancer is the 9th most frequent cancer worldwide. Its incidence is increasing. The pancreas is an infrequent site of metastasis in relation to any type of malignancy. In this study, we report our experience with a patient who has undergone a pancreaticoduodenectomy for metastatic bladder cancer. A 61-year-old male was admitted with jaundice and pancreas head mass. He underwent robot assisted-cystectomy and ileal conduit for bladder cancer 7 months ago. Initial diagnosis under the imaging study was a resectable pancreas head cancer. However, we did not rule-out a metastatic bladder cancer. He underwent a classic pancreaticoduodenectomy. Based on histologic findings and immunohistochemistry results, a pancreas tumor with 4.9-cm sized metastatic urothelial carcinoma was diagnosed. He experienced no complication. He was discharged 11 days after the surgery. Four cycles of gemcitabine and cisplatin were administered. He remained recurrence-free of tumors for 16 months. Although the benefit of pancreatectomy for patient survival has been reported for metastases from renal cell carcinoma, it is unknown for bladder cancer because of no report. We believe that curative resection for metastasis to pancreas of urothelial carcinoma might be helpful for its management.
Keyword
Figure
Reference
-
1. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. 2017; Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 71:96–108. DOI: 10.1016/j.eururo.2016.06.010. PMID: 27370177.
Article2. Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. 2013; Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 63:234–241. DOI: 10.1016/j.eururo.2012.07.033. PMID: 22877502.
Article3. Song W, Jeon HG. 2015; Incidence of kidney, bladder, and prostate cancers in Korea: an update. Korean J Urol. 56:422–428. DOI: 10.4111/kju.2015.56.6.422. PMID: 26078838. PMCID: PMC4462631.
Article4. Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD. 2011; Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol. 196:117–122. DOI: 10.2214/AJR.10.5036. PMID: 21178055.
Article5. Z'graggen K, Fernández-del Castillo C, Rattner DW, Sigala H, Warshaw AL. 1998; Metastases to the pancreas and their surgical extirpation. Arch Surg. 133:413–417. DOI: 10.1001/archsurg.133.4.413. PMID: 9565122.6. Chan E, Garg K, Stohr BA. 2020; Integrated immunohistochemical and molecular analysis improves diagnosis of high-grade carcinoma in the urinary bladder of patients with prior radiation therapy for prostate cancer. Mod Pathol. 33:1802–1810. DOI: 10.1038/s41379-020-0543-y. PMID: 32313185.
Article7. Bellizzi AM. 2020; An algorithmic immunohistochemical approach to define tumor type and assign site of origin. Adv Anat Pathol. 27:114–163. DOI: 10.1097/PAP.0000000000000256. PMID: 32205473. PMCID: PMC7700753.
Article8. Wilkerson ML, Lin F, Liu H, Cheng L. 2014; The application of immunohistochemical biomarkers in urologic surgical pathology. Arch Pathol Lab Med. 138:1643–1665. DOI: 10.5858/arpa.2014-0078-RA. PMID: 25427043.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biliary Tract and Pancreas: Survival and Recurrence Pattern after Curative Resection of Pancreatic Cancer
- Therapeutic approach to non-curative resection after endoscopic treatment in early gastric cancer
- Metastatic Penile Cancer Originated from Pancreas
- Surgical Treatment and Outcomes of Primary Duodenal Adenocarcinoma
- Curative Resection of Inoperable, Locally Advanced Gastric Cancer after Neoadjuvant Chemotherapy with Taxotere and Cisplatin