J Korean Med Sci.
2021 Aug;36(30):e193. 10.3346/jkms.2021.36.e193.
Association between Parental Cotinineverified Smoking Status and Childhood Asthma: a Population-based Nationally Representative Analysis
- Affiliations
-
- 1Department of Pediatrics, Kosin University Gospel Hospital, Kosin University School of Medicine, Busan, Korea
- 2Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University School of Medicine, Busan, Korea
- KMID: 2519194
- DOI: http://doi.org/10.3346/jkms.2021.36.e193
Abstract
- Background
Environmental tobacco smoke exposure due to parents is a modifiable risk factor for childhood asthma, but many studies have evaluated parental smoking using selfreported data. Therefore, we aimed to analyze the relationship between parental cotinineverified smoking status and asthma in their children.
Methods
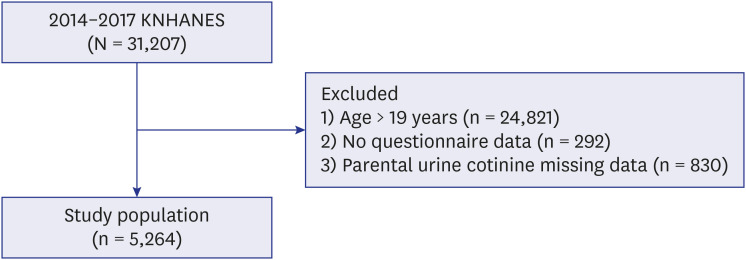
This population-based cross-sectional study used data from the Korean National Health and Nutrition Examination Survey from 2014 to 2017. Participants aged 0 to 18 years with complete self-reported physician-diagnosed childhood asthma and measurement of their parental urinary cotinine levels were included. Parental urinary cotinine-verified smoking status was defined using both urinary cotinine levels and self-report, as active, passive, and non-smoker. Sample weights were applied to all statistical analyses because of a complex, multistage and clustered survey design. Logistic regression model was used to analyze the relationship between childhood asthma and parental smoking.
Results
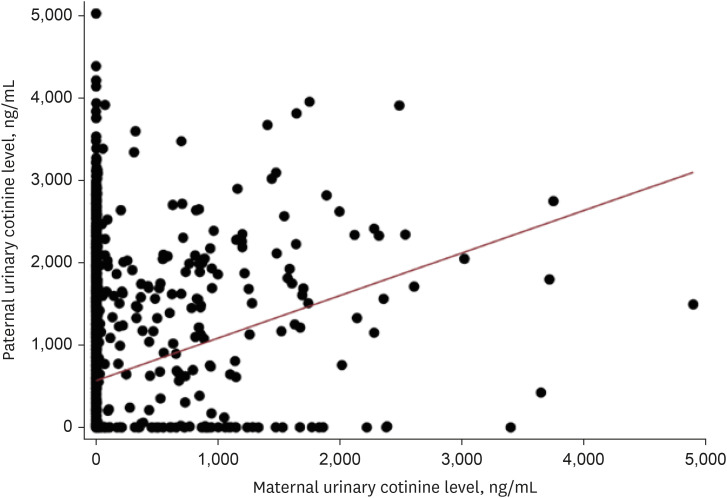
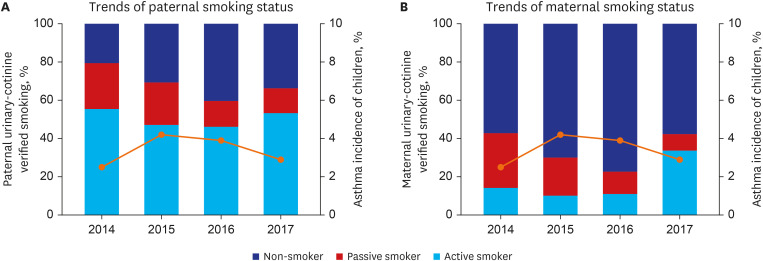
A total of 5,264 subjects aged < 19 years were included. The prevalence of asthma was 3.4%. The proportions of paternal and maternal urinary cotinine-verified active smokers during the study period were 50.4% and 16.9%, respectively. When parental urinary cotinine level increased, the proportion of parental low household income was increased (P < 0.001). There was no significant association between the parental urinary cotinine-verified smoking group and childhood asthma group. However, the adjusted odds ratios of childhood asthma in the middle and highest tertile of paternal urinary cotinine levels compared with those in lowest tertile were 1.95 (95% confidence interval [CI], 0.98–3.89) and 2.34 (95% CI, 1.21–4.54), respectively.
Conclusion
There seems to be a dose-related association between paternal urinary cotinine levels and the risk of childhood asthma. Because of the high rate of paternal smoking, further studies are needed to develop a targeted strategy to reduce parental smoking for childhood asthma.
Figure
Reference
-
1. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019; 7:246. PMID: 31275909.
Article2. Asher MI, García-Marcos L, Pearce NE, Strachan DP. Trends in worldwide asthma prevalence. Eur Respir J. 2020; 56(6):2002094. PMID: 32972987.
Article3. Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet. 2018; 391(10118):350–400. PMID: 28911920.
Article4. Bisgaard H, Bønnelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010; 126(2):187–197. PMID: 20688204.
Article5. Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. Lancet Respir Med. 2017; 5(3):224–234. PMID: 27666650.
Article6. Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ, et al. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol. 2006; 118(5):1040–1047. PMID: 17088127.
Article7. Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008; 372(9643):1058–1064. PMID: 18805334.
Article8. Limb SL, Brown KC, Wood RA, Wise RA, Eggleston PA, Tonascia J, et al. Adult asthma severity in individuals with a history of childhood asthma. J Allergy Clin Immunol. 2005; 115(1):61–66. PMID: 15637548.
Article9. Limb SL, Brown KC, Wood RA, Wise RA, Eggleston PA, Tonascia J, et al. Irreversible lung function deficits in young adults with a history of childhood asthma. J Allergy Clin Immunol. 2005; 116(6):1213–1219. PMID: 16337448.
Article10. Tai A, Tran H, Roberts M, Clarke N, Gibson AM, Vidmar S, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol. 2014; 133(6):1572–1578.e3. PMID: 24495434.
Article11. Global Initiative for Asthma. Global strategy for asthma management and prevention 2020. Updated 2020. Accessed January 10, 2021. https://ginasthma.org/.12. Zacharasiewicz A.Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Res. 2016; 2(3):00042-2016.
Article13. Wang B, Chen H, Chan YL, Wang G, Oliver BG. Why do intrauterine exposure to air pollution and cigarette smoke increase the risk of asthma? Front Cell Dev Biol. 2020; 8:38. PMID: 32117969.
Article14. Thacher JD, Gruzieva O, Pershagen G, Neuman Å, Wickman M, Kull I, et al. Pre- and postnatal exposure to parental smoking and allergic disease through adolescence. Pediatrics. 2014; 134(3):428–434. PMID: 25136039.
Article15. Kearley J, Silver JS, Sanden C, Liu Z, Berlin AA, White N, et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity. 2015; 42(3):566–579. PMID: 25786179.
Article16. Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to TH2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008; 122(6):1208–1214. PMID: 18926564.17. Lanckacker EA, Tournoy KG, Hammad H, Holtappels G, Lambrecht BN, Joos GF, et al. Short cigarette smoke exposure facilitates sensitisation and asthma development in mice. Eur Respir J. 2013; 41(5):1189–1199. PMID: 22903968.
Article18. Gangl K, Reininger R, Bernhard D, Campana R, Pree I, Reisinger J, et al. Cigarette smoke facilitates allergen penetration across respiratory epithelium. Allergy. 2009; 64(3):398–405. PMID: 19120070.
Article19. Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014; 134(3):499–507. PMID: 25171864.
Article20. Priest N, Roseby R, Waters E, Polnay A, Campbell R, Spencer N, et al. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2008; (4):CD001746. PMID: 18843622.
Article21. Silvestri M, Franchi S, Pistorio A, Petecchia L, Rusconi F. Smoke exposure, wheezing, and asthma development: a systematic review and meta-analysis in unselected birth cohorts. Pediatr Pulmonol. 2015; 50(4):353–362. PMID: 24648197.
Article22. Wang Z, May SM, Charoenlap S, Pyle R, Ott NL, Mohammed K, et al. Effects of secondhand smoke exposure on asthma morbidity and health care utilization in children: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2015; 115(5):396–401.e2. PMID: 26411971.
Article23. Man CN, Fathelrahman AI, Harn GL, Lajis R, Samin AS, Omar M, et al. Correlation between urinary nicotine, cotinine and self-reported smoking status among educated young adults. Environ Toxicol Pharmacol. 2009; 28(1):92–96. PMID: 21783987.
Article24. Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009; 11(1):12–24. PMID: 19246437.25. Kim Y, Choi YJ, Oh SW, Joh HK, Kwon H, Um YJ, et al. Discrepancy between self-reported and urine-cotinine verified smoking status among Korean male adults: analysis of health check-up data from a single private hospital. Korean J Fam Med. 2016; 37(3):171–176. PMID: 27274388.
Article26. Jhun HJ, Seo HG, Lee DH, Sung MW, Kang YD, Syn HC, et al. Self-reported smoking and urinary cotinine levels among pregnant women in Korea and factors associated with smoking during pregnancy. J Korean Med Sci. 2010; 25(5):752–757. PMID: 20436713.
Article27. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014; 43(1):69–77. PMID: 24585853.
Article28. Zuraimi MS, Tham KW, Chew FT, Ooi PL, David K. Home exposures to environmental tobacco smoke and allergic symptoms among young children in Singapore. Int Arch Allergy Immunol. 2008; 146(1):57–65. PMID: 18087162.
Article29. Zhang X, Johnson N, Carrillo G, Xu X. Decreasing trend in passive tobacco smoke exposure and association with asthma in U.S. children. Environ Res. 2018; 166:35–41. PMID: 29859939.
Article30. Lang JE, Tang M. Smoking: it's still a big problem in children with asthma. J Pediatr (Rio J). 2019; 95(5):506–508. PMID: 30590013.
Article31. Mitchell EA, Beasley R, Keil U, Montefort S, Odhiambo J. ISAAC Phase Three Study Group. The association between tobacco and the risk of asthma, rhinoconjunctivitis and eczema in children and adolescents: analyses from phase three of the ISAAC programme. Thorax. 2012; 67(11):941–949. PMID: 22693180.
Article32. Merianos AL, Jandarov RA, Mahabee-Gittens EM. Secondhand smoke exposure and pediatric healthcare visits and hospitalizations. Am J Prev Med. 2017; 53(4):441–448. PMID: 28532658.
Article33. Lee A, Lee SY, Lee KS. Association of secondhand smoke exposure with allergic multimorbidity in Korean adolescents. Sci Rep. 2020; 10(1):16409. PMID: 33009485.
Article34. Lannerö E, Wickman M, van Hage M, Bergström A, Pershagen G, Nordvall L. Exposure to environmental tobacco smoke and sensitisation in children. Thorax. 2008; 63(2):172–176. PMID: 18089631.
Article35. Robays LJ, Maes T, Joos GF, Vermaelen KY. Between a cough and a wheeze: dendritic cells at the nexus of tobacco smoke-induced allergic airway sensitization. Mucosal Immunol. 2009; 2(3):206–219. PMID: 19262504.
Article36. Rumold R, Jyrala M, Diaz-Sanchez D. Secondhand smoke induces allergic sensitization in mice. J Immunol. 2001; 167(8):4765–4770. PMID: 11591808.
Article37. Trimble NJ, Botelho FM, Bauer CM, Fattouh R, Stämpfli MR. Adjuvant and anti-inflammatory properties of cigarette smoke in murine allergic airway inflammation. Am J Respir Cell Mol Biol. 2009; 40(1):38–46. PMID: 18635815.
Article38. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, Celedón JC. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016; 4(6):1111–1122. PMID: 27286779.
Article39. Simons E, To T, Moineddin R, Stieb D, Dell SD. Maternal second-hand smoke exposure in pregnancy is associated with childhood asthma development. J Allergy Clin Immunol Pract. 2014; 2(2):201–207.e3. PMID: 24607049.
Article40. Gopal SH, Mukherjee S, Das SK. Direct and second hand cigarette smoke exposure and development of childhood asthma. J Environ Health Sci. 2016; 2(6):1–5.41. Tsai CH, Huang JH, Hwang BF, Lee YL. Household environmental tobacco smoke and risks of asthma, wheeze and bronchitic symptoms among children in Taiwan. Respir Res. 2010; 11(1):11. PMID: 20113468.
Article42. Pattenden S, Antova T, Neuberger M, Nikiforov B, De Sario M, Grize L, et al. Parental smoking and children's respiratory health: independent effects of prenatal and postnatal exposure. Tob Control. 2006; 15(4):294–301. PMID: 16885578.
Article43. Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001; 163(2):429–436. PMID: 11179118.
Article44. Booalayan H, Abdualrasool M, Al-Shanfari S, Boujarwa A, Al-Mukaimi A, Alkandery O, et al. Exposure to environmental tobacco smoke and prevalence of asthma among adolescents in a middle eastern country. BMC Public Health. 2020; 20(1):1210. PMID: 32770990.
Article45. Korea Centers for Disease Control and Prevention. Korea National Health and Nutrition Examination Survey: fact sheet 2020. Updated 2020. Accessed January 21, 2021. https://policy.nl.go.kr/search/searchDetail.do?rec_key=SH2_PLC20200255775.46. Gunter R, Szeto E, Jeong SH, Suh S, Waters AJ. Cigarette smoking in South Korea: a narrative review. Korean J Fam Med. 2020; 41(1):3–13. PMID: 31189304.
Article47. World Health Organization. WHO report on the global tobacco use trends 2019. Updated 2019. Accessed January 24, 2021. https://www.who.int/news/item/19-12-2019-who-launches-new-report-on-global-tobacco-use-trends.48. Waldron I. Patterns and causes of gender differences in smoking. Soc Sci Med. 1991; 32(9):989–1005. PMID: 2047903.
Article49. Jarvis MJ, Feyerabend C. Recent trends in children's exposure to second-hand smoke in England: cotinine evidence from the Health Survey for England. Addiction. 2015; 110(9):1484–1492. PMID: 26061741.
Article50. Reitan T, Callinan S. Changes in smoking rates among pregnant women and the general female population in Australia, Finland, Norway, and Sweden. Nicotine Tob Res. 2017; 19(3):282–289. PMID: 27613884.
Article51. American Lung Association. Tobacco trends brief. Updated 2020. Accessed January 24, 2021. https://www.lung.org/research/trends-in-lung-disease/tobacco-trends-brief/overall-tobacco-trends.52. Berman T, Barnett-Itzhaki Z, Axelrod R, Keinan-Boker L, Shimony T, Goldsmith R, et al. Socioeconomic inequalities in exposure to environmental tobacco smoke in children in Israel. Environ Int. 2018; 121(Pt 1):643–648. PMID: 30316179.
Article53. Kuntz B, Lampert T. Social disparities in parental smoking and young children's exposure to secondhand smoke at home: a time-trend analysis of repeated cross-sectional data from the German KiGGS study between 2003-2006 and 2009-2012. BMC Public Health. 2016; 16(1):485. PMID: 27277721.
Article54. Biagini Myers JM, Khurana Hershey GK, Deka R, Burkle JW, Levin LS, Bernstein DI, et al. Asking the right questions to ascertain early childhood secondhand smoke exposures. J Pediatr. 2012; 160(6):1050–1051. PMID: 22494871.
Article55. Jain RB. Trends in exposure to second hand smoke at home among children and nonsmoker adolescents. Sci Total Environ. 2016; 542(Pt A):144–152. PMID: 26519575.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The association between smoking and asthma
- The Influence of Passive Smoking on Asthma and Allergic Disease in Preschool-Aged Children
- The Importance of Parental Attitudes and Behavior upon Adolescent Smoking Behavior
- The association between maternal depression and childhood allergic diseases: an analysis of the Fifth Korea National Health and Nutrition Examination Survey (2010-2012)
- Environmental Tobacco Smoking, Parental Allergy History and Pediatric Asthma and Wheezing