Clin Endosc.
2021 Jul;54(4):477-487. 10.5946/ce.2021.160.
Application of Current Image-Enhanced Endoscopy in Gastric Diseases
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Hwasun, Korea
- KMID: 2518849
- DOI: http://doi.org/10.5946/ce.2021.160
Abstract
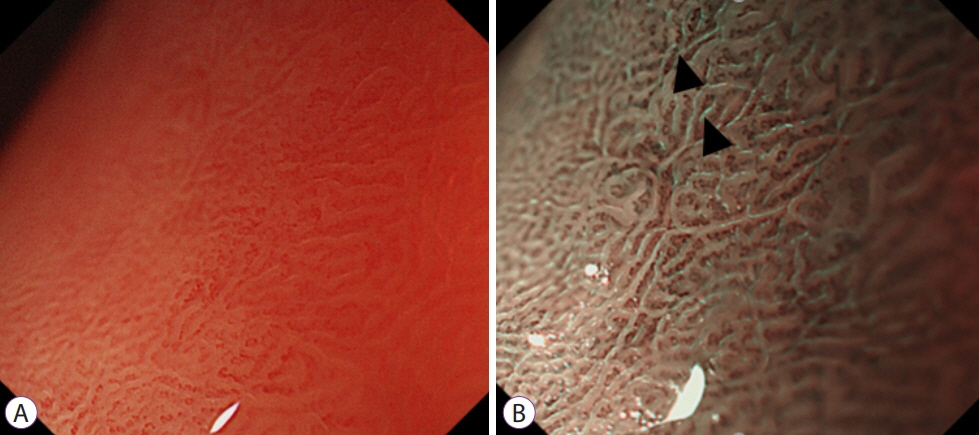
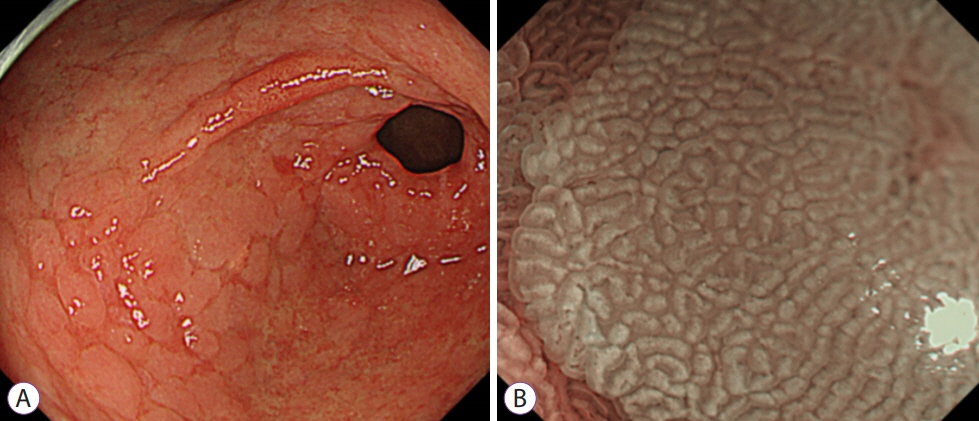
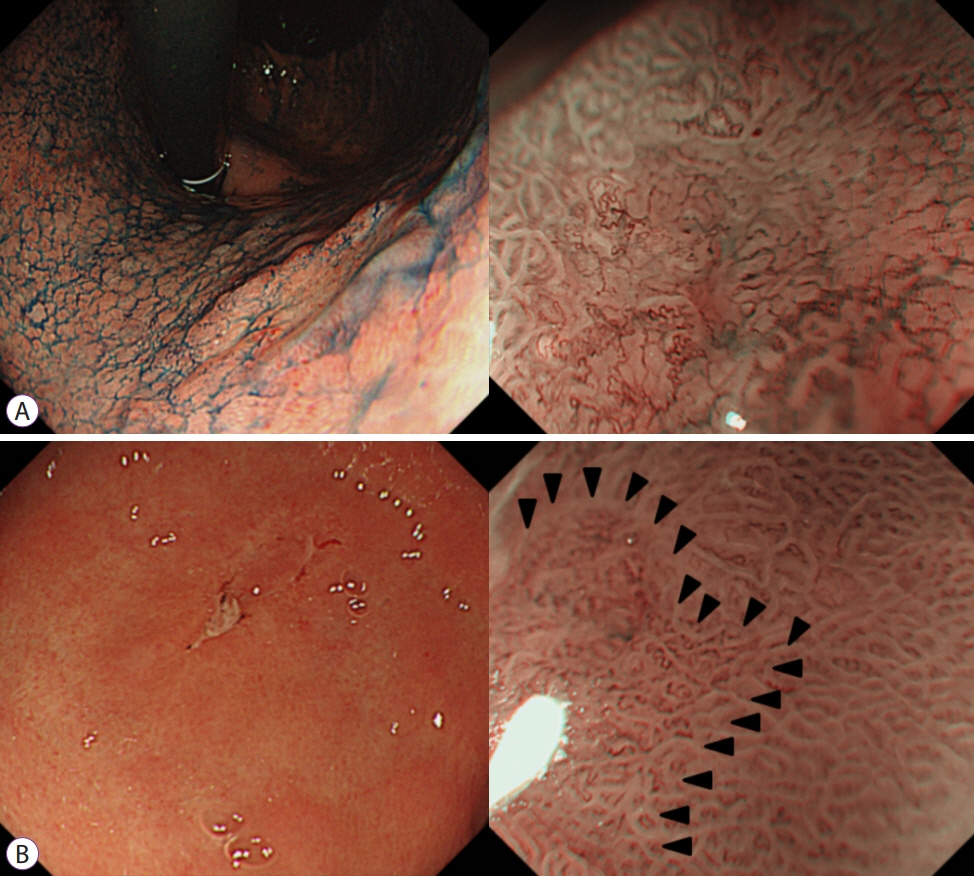
- Image-enhanced endoscopy (IEE) plays an integral role in endoscopic diagnosis and treatment. IEE enables an early and accurate detection of cancer and characterization of lesions prior to therapeutic decisions. Ideal IEE can serve as an optical or digital chromoscopic endoscopy, as well as an optical biopsy that predicts exact histopathology. Several IEE modalities have recently been developed and are used in the clinical field. The stomach is a challenging organ for imaging because of its complex secretion function and status of Helicobacter pylori infection. Therefore, understanding the current IEE modalities for their clinical applicability in an evidence-based approach is warranted. Along with technology refinements, the new paradigm will be available for the diagnosis of gastric cancer or other conditions in the stomach in the near future.
Figure
Cited by 1 articles
-
위바닥샘형 선암의 내시경 절제에 대한 증례 시리즈
Hwa Jin Lee, Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyungbin Kim
Korean J Gastroenterol. 2023;81(6):259-264. doi: 10.4166/kjg.2023.019.
Reference
-
1. Muto M, Katada C, Sano Y, Yoshida S. Narrow band imaging: a new diagnostic approach to visualize angiogenesis in superficial neoplasia. Clin Gastroenterol Hepatol. 2005; 3:S16–S20.
Article2. Capelle LG, Haringsma J, de Vries AC, et al. Narrow band imaging for the detection of gastric intestinal metaplasia and dysplasia during surveillance endoscopy. Dig Dis Sci. 2010; 55:3442–3448.
Article3. Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013; 26:11–22.4. Qi Q, Guo C, Ji R, Li Z, Zuo X, Li Y. Diagnostic performance of magnifying endoscopy for Helicobacter pylori infection: a meta-analysis. PLoS One. 2016; 11:e0168201.
Article5. Anagnostopoulos GK, Yao K, Kaye P, et al. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007; 39:202–207.
Article6. Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019; 51:365–388.
Article7. Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006; 38:819–824.
Article8. Pimentel-Nunes P, Dinis-Ribeiro M, Soares JB, et al. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy. 2012; 44:236–246.
Article9. Yao K, Iwashita A, Tanabe H, et al. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008; 68:574–580.
Article10. Matsushita M, Mori S, Uchida K, Nishio A, Okazaki K. “White opaque substance” and “light blue crest” within gastric flat tumors or intestinal metaplasia: same or different signs? Gastrointest Endosc. 2009; 70:402. author reply 402-403.
Article11. Yao K, Oishi T. Microgastroscopic findings of mucosal microvascular architecture as visualized by magnifying endoscopy. Dig Endosc. 2001; 13:S27–S33.
Article12. Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004; 36:1080–1084.
Article13. Kiyotoki S, Nishikawa J, Satake M, et al. Usefulness of magnifying endoscopy with narrow-band imaging for determining gastric tumor margin. J Gastroenterol Hepatol. 2010; 25:1636–1641.
Article14. Yagi K, Nakamura A, Sekine A, Umezu H. Magnifying endoscopy with narrow band imaging for early differentiated gastric adenocarcinoma. Dig Endosc. 2008; 20:115–122.
Article15. Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009; 41:462–467.
Article16. Yao K. Clinical application of magnifying endoscopy with narrow-band imaging in the stomach. Clin Endosc. 2015; 48:481–490.
Article17. Ezoe Y, Muto M, Uedo N, et al. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011; 141:2017–2025.e3.
Article18. Hu YY, Lian QW, Lin ZH, Zhong J, Xue M, Wang LJ. Diagnostic performance of magnifying narrow-band imaging for early gastric cancer: A meta-analysis. World J Gastroenterol. 2015; 21:7884–7894.
Article19. Yao K, Iwashita A, Nambu M, et al. Nature of white opaque substance in gastric epithelial neoplasia as visualized by magnifying endoscopy with narrow-band imaging. Dig Endosc. 2012; 24:419–425.
Article20. Kaise M, Kato M, Urashima M, et al. Magnifying endoscopy combined with narrow-band imaging for differential diagnosis of superficial depressed gastric lesions. Endoscopy. 2009; 41:310–315.
Article21. Nonaka K, Arai S, Ban S, et al. Prospective study of the evaluation of the usefulness of tumor typing by narrow band imaging for the differential diagnosis of gastric adenoma and well-differentiated adenocarcinoma. Dig Endosc. 2011; 23:146–152.
Article22. Doyama H, Yoshida N, Tsuyama S, et al. The “white globe appearance” (WGA): a novel marker for a correct diagnosis of early gastric cancer by magnifying endoscopy with narrow-band imaging (M-NBI). Endosc Int Open. 2015; 3:E120–E124.
Article23. Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc. 2016; 28:379–393.
Article24. Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004; 36:1080–1084.
Article25. Yokoyama A, Inoue H, Minami H, et al. Novel narrow-band imaging magnifying endoscopic classification for early gastric cancer. Dig Liver Dis. 2010; 42:704–708.
Article26. Kobayashi M, Takeuchi M, Ajioka Y, et al. Mucin phenotype and narrow-band imaging with magnifying endoscopy for differentiated-type mucosal gastric cancer. J Gastroenterol. 2011; 46:1064–1070.
Article27. Li HY, Ge ZZ, Fujishiro M, Li XB. Current clinical applications of magnifying endoscopy with narrow band imaging in the stomach. Diagn Ther Endosc. 2012; 2012:271914.
Article28. Nagahama T, Yao K, Maki S, et al. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc. 2011; 74:1259–1267.
Article29. Okada K, Fujisaki J, Kasuga A, et al. Diagnosis of undifferentiated type early gastric cancers by magnification endoscopy with narrow-band imaging. J Gastroenterol Hepatol. 2011; 26:1262–1269.
Article30. Nonaka K, Ishikawa K, Shimizu M, et al. Education and imaging. Gastrointestinal: gastric mucosa-associated lymphoma presented with unique vascular features on magnified endoscopy combined with narrow-band imaging. J Gastroenterol Hepatol. 2009; 24:1697.31. Ono S, Kato M, Ono Y, et al. Target biopsy using magnifying endoscopy in clinical management of gastric mucosa-associated lymphoid tissue lymphoma. J Gastroenterol Hepatol. 2011; 26:1133–1138.
Article32. Okubo M, Tahara T, Shibata T, et al. Changes in gastric mucosal patterns seen by magnifying NBI during H. pylori eradication. J Gastroenterol. 2011; 46:175–182.33. Ono S, Kato M, Ono Y, et al. Characteristics of magnified endoscopic images of gastric extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue, including changes after treatment. Gastrointest Endosc. 2008; 68:624–631.
Article34. Lee TH, Chung IK, Park JY, et al. Usefulness of magnifying endoscopy in post-endoscopic resection scar for early gastric neoplasm: a prospective short-term follow-up endoscopy study. World J Gastroenterol. 2009; 15:349–355.
Article35. Osawa H, Yamamoto H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc. 2014; 26 Suppl 1:105–115.
Article36. Jang JY. The past, present, and future of image-enhanced endoscopy. Clin Endosc. 2015; 48:466–475.
Article37. Osawa H, Miura Y, Takezawa T, et al. Linked color imaging and blue laser imaging for upper gastrointestinal screening. Clin Endosc. 2018; 51:513–526.
Article38. Jeon SR, Cho WY, Jin SY, Cheon YK, Choi SR, Cho JY. Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. Gastrointest Endosc. 2011; 74:772–780.
Article39. Li WB, Zuo XL, Li CQ, et al. Diagnostic value of confocal laser endomicroscopy for gastric superficial cancerous lesions. Gut. 2011; 60:299–306.
Article40. Zhang HP, Yang S, Chen WH, Hu TT, Lin J. The diagnostic value of confocal laser endomicroscopy for gastric cancer and precancerous lesions among Asian population: a system review and meta-analysis. Scand J Gastroenterol. 2017; 52:382–388.
Article41. Park JC, Park Y, Kim HK, et al. Probe-based confocal laser endomicroscopy in the margin delineation of early gastric cancer for endoscopic submucosal dissection. J Gastroenterol Hepatol. 2017; 32:1046–1054.
Article42. Park CH, Kim H, Jo JH, et al. Role of probe-based confocal laser endomicroscopy-targeted biopsy in the molecular and histopathological study of gastric cancer. J Gastroenterol Hepatol. 2019; 34:84–91.
Article43. Kumagai Y, Kawada K, Takubo K, Ishida H. Ultra-high magnification endoscopy (endocytoscopy system) for examination of esophageal lesions. Gastroenterological Endoscopy. 2017; 59:207–218.44. Kumagai Y, Takubo K, Kawada K, et al. A newly developed continuous zoom-focus endocytoscope. Endoscopy. 2017; 49:176–180.
Article45. Kaise M, Kimura R, Nomura K, et al. Accuracy and concordance of endocytoscopic atypia for the diagnosis of gastric cancer. Endoscopy. 2014; 46:827–832.
Article46. Sato H, Inoue H, Ikeda H, et al. In vivo gastric mucosal histopathology using endocytoscopy. World J Gastroenterol. 2015; 21:5002–5008.47. Abad MRA, Inoue H, Ikeda H, et al. Utilizing fourth-generation endocytoscopy and the “enlarged nuclear sign” for in vivo diagnosis of early gastric cancer. Endosc Int Open. 2019; 7:E1002–E1007.
Article48. Sato H, Inoue H, Hayee B, et al. In vivo histopathology using endocytoscopy for non-neoplastic changes in the gastric mucosa: a prospective pilot study (with video). Gastrointest Endosc. 2015; 81:875–881.49. Kutsukawa M, Kudo SE, Ikehara N, et al. Efficiency of endocytoscopy in differentiating types of serrated polyps. Gastrointest Endosc. 2014; 79:648–656.
Article50. Kimura S, Inoue H, Sato Y, et al. Ex vivo visualization of Helicobacter pylori using an endocytoscopic probe. Biomed Res. 2006; 27:255–257.
Article51. Tsurudome I, Miyahara R, Funasaka K, et al. In vivo histological diagnosis for gastric cancer using endocytoscopy. World J Gastroenterol. 2017; 23:6894–6901.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is Image-Enhanced Endoscopy Useful for the Diagnosis and Treatment of Gastrointestinal Tumor?
- The Past, Present, and Future of Image-Enhanced Endoscopy
- Application of artificial intelligence for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging
- Image-Enhanced Endoscopy and Its Corresponding Histopathology in the Stomach
- Current Status of Image-Enhanced Endoscopy for Early Identification of Esophageal Neoplasms