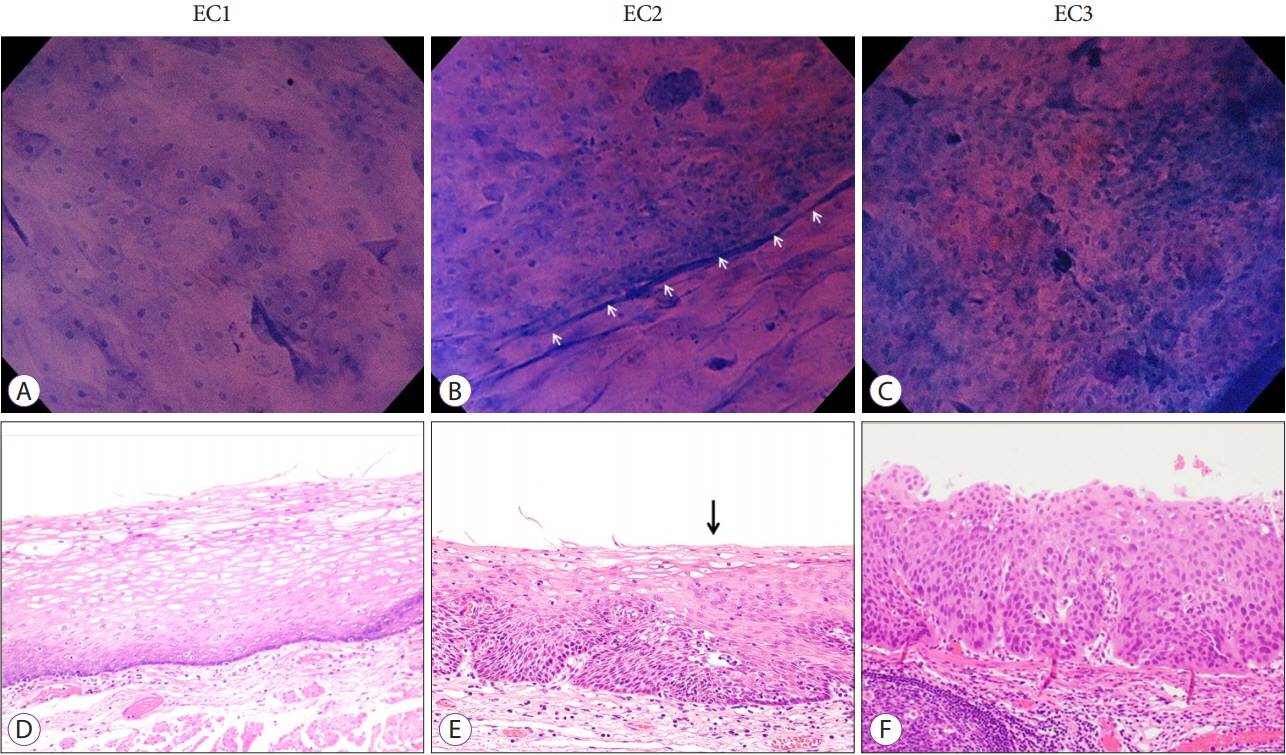
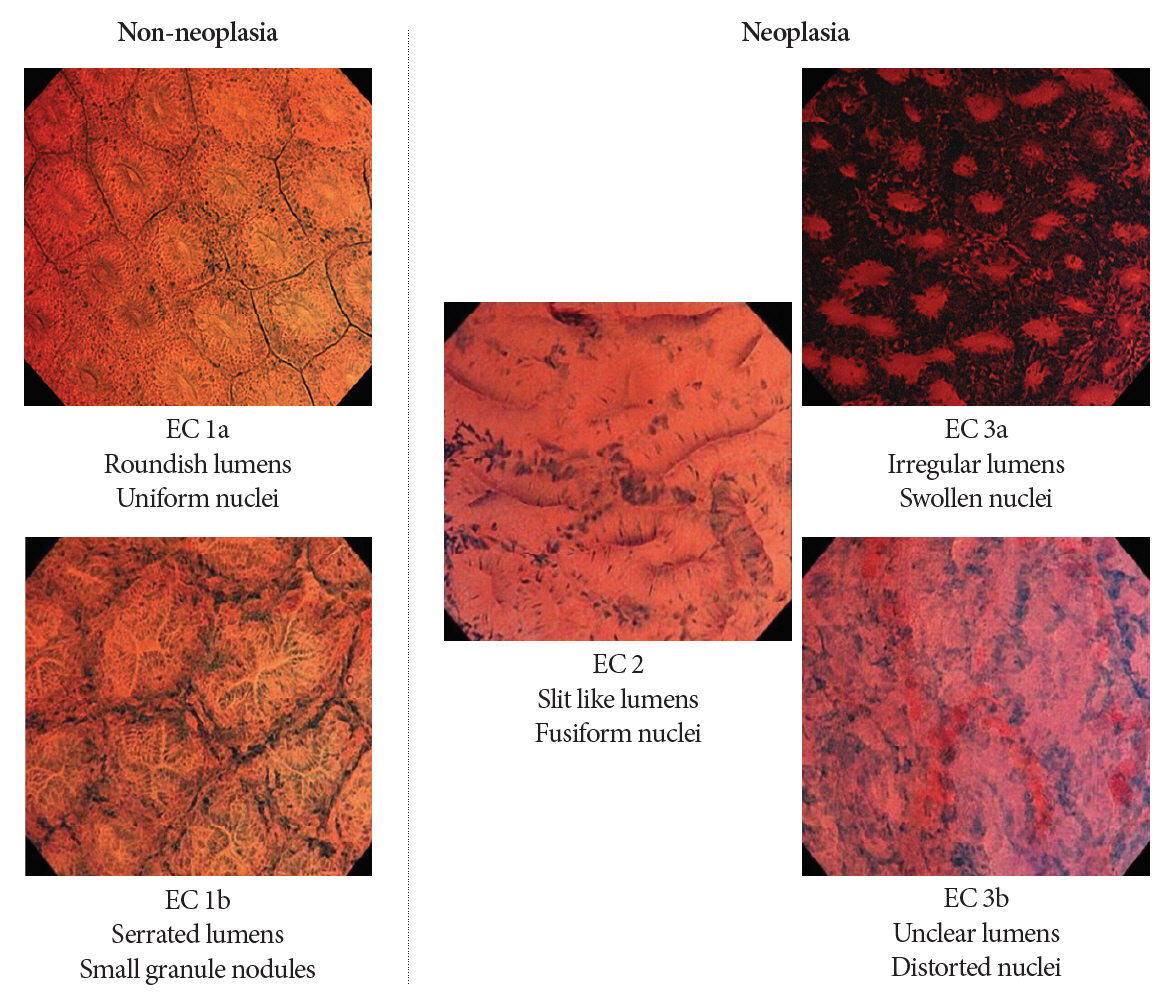
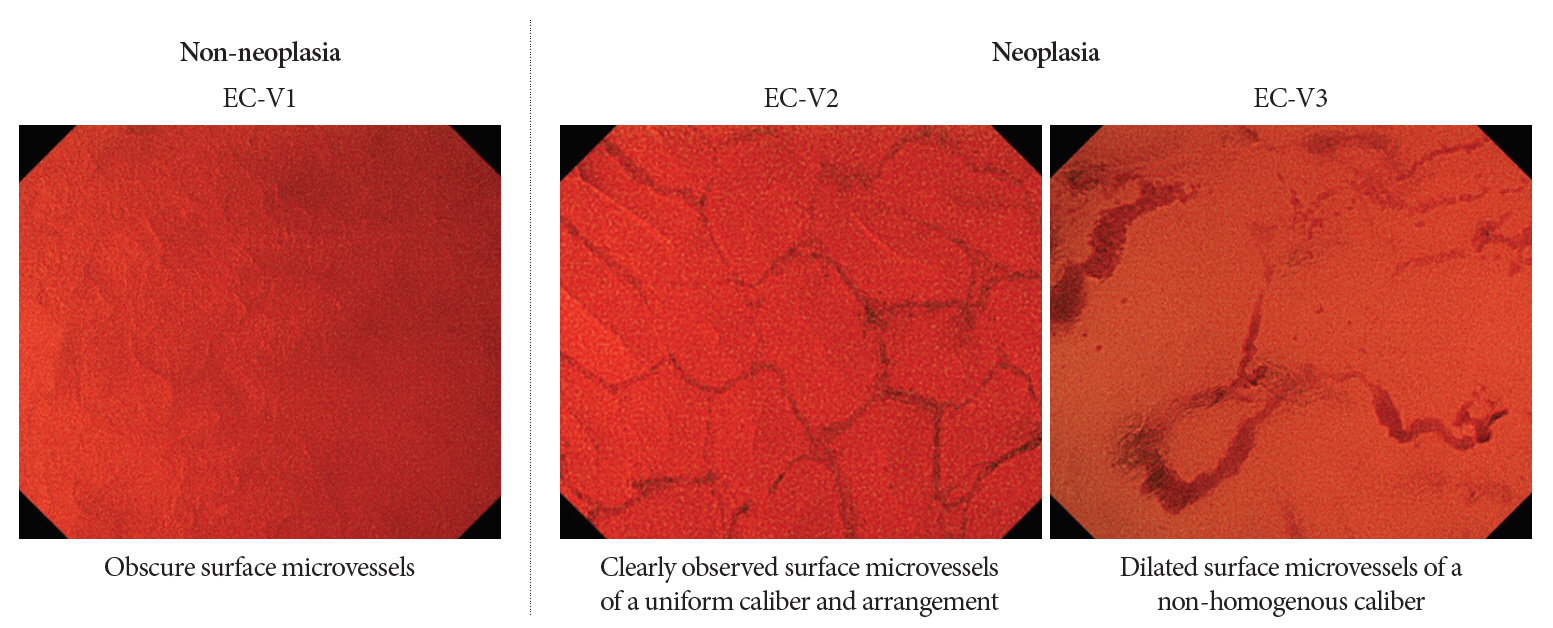
Clin Endosc.
2021 Jul;54(4):455-463. 10.5946/ce.2021.165.
Clinical Efficacy of Endocytoscopy for Gastrointestinal Endoscopy
- Affiliations
-
- 1Digestive Disease Center, Showa University Northern Yokohama Hospital, Yokohama, Japan
- 2Clinical Effectiveness Research Group, Institute of Health and Society, University of Oslo, Oslo, Norway
- 3Digestive Disease Center, Showa University Koto Toyosu Hospital, Tokyo, Japan
- KMID: 2518847
- DOI: http://doi.org/10.5946/ce.2021.165
Abstract
- Endocytoscopy (EC) is a contact-type optical endoscope that allows in vivo cellular observation during gastrointestinal endoscopy and is now commercially available not only in Japan but also in Asian, European Union, and Middle Eastern countries. EC helps conduct a highly accurate pathological prediction without biopsy. Initially, EC was reported to be effective for esophageal diseases. Subsequently, its efficacy for stomach and colorectal diseases has been reported. In this narrative review, we searched for clinical studies that investigated the efficacy of EC. EC seems to accurately diagnose gastrointestinal diseases without biopsy. Most of the studies aimed to clarify the relationship between endocytoscopic findings of gastrointestinal neoplasia and pathological diagnosis. Some studies have investigated non-epithelial lesions or diseases, such as inflammatory bowel disease or infectious diseases. However, there are few high-level pieces of evidence, such as randomized trials; thus, further studies are needed.
Keyword
Figure
Reference
-
1. Inoue H, Kazawa T, Sato Y, et al. In vivo observation of living cancer cells in the esophagus, stomach, and colon using catheter-type contact endoscope, “Endo-Cytoscopy system”. Gastrointest Endosc Clin N Am. 2004; 14:589–594. x-xi.
Article2. Kumagai Y, Monma K, Kawada K. Magnifying chromoendoscopy of the esophagus: in-vivo pathological diagnosis using an endocytoscopy system. Endoscopy. 2004; 36:590–4.
Article3. Sasajima K, Kudo SE, Inoue H, et al. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc. 2006; 63:1010–1017.
Article4. Kumagai Y, Yamamoto E, Higashi M, et al. Endocytoscopic observation with methylene blue staining for duodenal neoplasms associated with familial adenomatous polyposis. Sci Rep. 2020; 10:19221.
Article5. Kudo SE, Maeda Y, Ogata N, et al. Combined endocytoscopy with pit pattern diagnosis in ulcerative colitis-associated neoplasia: pilot study. Dig Endosc. 2021; Feb. 28. [Epub]. https://doi.org/10.1111/den.13964.
Article6. Ichimasa K, Kudo SE, Mori Y, et al. Double staining with crystal violet and methylene blue is appropriate for colonic endocytoscopy: an in vivo prospective pilot study. Dig Endosc. 2014; 26:403–408.7. Minami H, Inoue H, Yokoyama A, et al. Recent advancement of observing living cells in the esophagus using CM double staining: endocytoscopic atypia classification. Dis Esophagus. 2012; 25:235–241.
Article8. Inoue H, Sasajima K, Kaga M, et al. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy. 2006; 38:891–895.
Article9. Kumagai Y, Kawada K, Yamazaki S, et al. Endocytoscopic observation for esophageal squamous cell carcinoma: can biopsy histology be omitted? Dis Esophagus. 2009; 22:505–512.
Article10. Kumagai Y, Kawada K, Yamazaki S, et al. Current status and limitations of the newly developed endocytoscope GIF-Y0002 with reference to its diagnostic performance for common esophageal lesions. J Dig Dis. 2012; 13:393–400.
Article11. Kumagai Y, Kawada K, Higashi M, et al. Endocytoscopic observation of various esophageal lesions at ×600: can nuclear abnormality be recognized? Dis Esophagus. 2015; 28:269–275.
Article12. Shimamura Y, Inoue H, Rodriguez de Santiago E, et al. Diagnostic yield of fourth-generation endocytoscopy for esophageal squamous lesions using a modified endocytoscopic classification. Dig Endosc. 2020; Dec. 18. [Epub]. https://doi.org/10.1111/den.13914.
Article13. Eleftheriadis N, Inoue H, Ikeda H, et al. Endocytoscopic visualization of squamous cell islands within Barrett’s epithelium. World J Gastrointest Endosc. 2013; 5:174–179.
Article14. Tomizawa Y, Iyer PG, Wongkeesong LM, et al. Assessment of the diagnostic performance and interobserver variability of endocytoscopy in Barrett’s esophagus: a pilot ex-vivo study. World J Gastroenterol. 2013; 19:8652–8658.15. Eberl T, Jechart G, Probst A, et al. Can an endocytoscope system (ECS) predict histology in neoplastic lesions? Endoscopy. 2007; 39:497–501.
Article16. Isomoto H, Matsushima K, Hayashi T, et al. Endocytoscopic findings of lymphomas of the stomach. BMC Gastroenterol. 2013; 13:174.
Article17. Chiu PWY, Ng EKW, To KF, et al. Recognition of goblet cells upon endocytoscopy indicates the presence of gastric intestinal metaplasia. Dig Endosc. 2014; 26:52–56.
Article18. Kaise M, Kimura R, Nomura K, et al. Accuracy and concordance of endocytoscopic atypia for the diagnosis of gastric cancer. Endoscopy. 2014; 46:827–832.
Article19. Kaise M, Ohkura Y, Iizuka T, et al. Endocytoscopy is a promising modality with high diagnostic accuracy for gastric cancer. Endoscopy. 2015; 47:19–25.
Article20. Sato H, Inoue H, Hayee B, et al. In vivo histopathology using endocytoscopy for non-neoplastic changes in the gastric mucosa: a prospective pilot study (with video). Gastrointest Endosc. 2015; 81:875–881.21. Pohl H, Rösch T, Tanczos BT, Rudolph B, Schlüns K, Baumgart DC. Endocytoscopy for the detection of microstructural features in adult patients with celiac sprue: a prospective, blinded endocytoscopy-conventional histology correlation study. Gastrointest Endosc. 2009; 70:933–941.
Article22. Matysiak-Budnik T, Coron E, Mosnier J-F, Le Rhun M, Inoue H, Galmiche J-P. In vivo real-time imaging of human duodenal mucosal structures in celiac disease using endocytoscopy. Endoscopy. 2010; 42:191–196.
Article23. Goda K, Dobashi A, Yoshimura N, et al. Dye solution optimizing staining conditions for in vivo endocytoscopy for normal villi and superficial epithelial tumors in the duodenum. Ann Gastroenterol. 2019; 32:378–386.
Article24. Kodashima S, Fujishiro M, Takubo K, et al. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy. 2006; 38:1115–1121.
Article25. Cipolletta L, Bianco MA, Rotondano G, et al. Endocytoscopy can identify dysplasia in aberrant crypt foci of the colorectum: a prospective in vivo study. Endoscopy. 2009; 41:129–132.
Article26. Kudo SE, Wakamura K, Ikehara N, Mori Y, Inoue H, Hamatani S. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy. 2011; 43:869–875.
Article27. Mori Y, Kudo SE, Ikehara N, et al. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: a prospective randomized noninferiority trial. Endoscopy. 2013; 45:98–105.
Article28. Utsumi T, Sano Y, Iwatate M, et al. Prospective real-time evaluation of diagnostic performance using endocytoscopy in differentiating neoplasia from non-neoplasia for colorectal diminutive polyps (≤5 mm). World J Gastrointest Oncol. 2018; 10:96–102.29. Kutsukawa M, Kudo SE, Ikehara N, et al. Efficiency of endocytoscopy in differentiating types of serrated polyps. Gastrointest Endosc. 2014; 79:648–656.
Article30. Sugihara Y, Kudo SE, Miyachi H, et al. In vivo detection of desmoplastic reaction using endocytoscopy: a new diagnostic marker of submucosal or more extensive invasion in colorectal carcinoma. Mol Clin Oncol. 2017; 6:291–295.
Article31. Sako T, Kudo SE, Miyachi H, et al. A novel ability of endocytoscopy to diagnose histological grade of differentiation in T1 colorectal carcinomas. Endoscopy. 2018; 50:69–74.
Article32. Kudo T, Kudo SE, Wakamura K, et al. Diagnostic performance of endocytoscopy for evaluating the invasion depth of different morphological types of colorectal tumors. Dig Endosc. 2015; 27:754–761.
Article33. Kudo T, Kudo SE, Mori Y, et al. Classification of nuclear morphology in endocytoscopy of colorectal neoplasms. Gastrointest Endosc. 2017; 85:628–638.
Article34. Kudo T, Suzuki K, Mori Y, et al. Endocytoscopy for the differential diagnosis of colorectal low-grade adenoma: a novel possibility for the “resect and discard” strategy. Gastrointest Endosc. 2020; 91:676–683.
Article35. Kudo SE, Misawa M, Wada Y, et al. Endocytoscopic microvasculature evaluation is a reliable new diagnostic method for colorectal lesions (with video). Gastrointest Endosc. 2015; 82:912–923.
Article36. Nakamura H, Kudo SE, Misawa M, et al. Evaluation of microvascular findings of deeply invasive colorectal cancer by endocytoscopy with narrow-band imaging. Endosc Int Open. 2016; 4:E1280–E1285.
Article37. Bessho R, Kanai T, Hosoe N, et al. Correlation between endocytoscopy and conventional histopathology in microstructural features of ulcerative colitis. J Gastroenterol. 2011; 46:1197–1202.
Article38. Nakazato Y, Naganuma M, Sugimoto S, et al. Endocytoscopy can be used to assess histological healing in ulcerative colitis. Endoscopy. 2017; 49:560–563.
Article39. Neumann H, Vieth M, Neurath MF, Atreya R. Endocytoscopy allows accurate in vivo differentiation of mucosal inflammatory cells in IBD: a pilot study. Inflamm Bowel Dis. 2013; 19:356–362.40. Maeda Y, Ohtsuka K, Kudo SE, et al. Endocytoscopic narrow-band imaging efficiency for evaluation of inflammatory activity in ulcerative colitis. World J Gastroenterol. 2015; 21:2108–2115.
Article41. Nishiyama S, Oka S, Tanaka S, et al. Clinical usefulness of endocytoscopy in the remission stage of ulcerative colitis: a pilot study. J Gastroenterol. 2015; 50:1087–1093.
Article42. Ueda N, Isomoto H, Ikebuchi Y, et al. Endocytoscopic classification can be predictive for relapse in ulcerative colitis. Medicine (Baltimore). 2018; 97:e0107.
Article43. Maeda Y, Kudo SE, Ogata N, et al. Endocytoscopic intramucosal capillary network changes and crypt architecture abnormalities can predict relapse in patients with an ulcerative colitis Mayo endoscopic score of 1. Dig Endosc. 2020; 32:1082–1091.
Article44. Kudo SE, Misawa M, Mori Y, et al. Artificial intelligence-assisted system improves endoscopic identification of colorectal neoplasms. Clin Gastroenterol Hepatol. 2020; 18:1874–1881.e2.
Article45. Maeda Y, Kudo SE, Mori Y, et al. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc. 2019; 89:408–415.
Article46. Misawa M, Kudo SE, Mori Y, et al. Artificial intelligence-assisted polyp detection for colonoscopy: initial experience. Gastroenterology. 2018; 154:2027–2029.e3.
Article47. Mori Y, Kudo SE, Misawa M, et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: a prospective study. Ann Intern Med. 2018; 169:357–366.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application and Efficacy of Super-Magnifying Endoscopy for the Lower Intestinal Tract
- Role of Advanced Endoscopic Imaging Techniques in the Management of Inflammatory Bowel Disease
- Introduction to Starting Upper Gastrointestinal Endoscopy: Proper Insertion, Complete Observation, and Appropriate Photographing
- Usefulness of an Overtube Device in Gastrointestinal Endoscopy
- Observable Laryngopharyngeal Lesions during the Upper Gastrointestinal Endoscopy