Nutr Res Pract.
2021 Aug;15(4):431-443. 10.4162/nrp.2021.15.4.431.
Efficacy of nobiletin in improving hypercholesterolemia and nonalcoholic fatty liver disease in high-cholesterol diet-fed mice
- Affiliations
-
- 1Department of Food Science and Nutrition, Kyungpook National University, Daegu 41566, Korea
- 2Department of Food Science and Nutrition, Pukyong National University, Busan 48513, Korea
- KMID: 2518751
- DOI: http://doi.org/10.4162/nrp.2021.15.4.431
Abstract
- BACKGROUND/OBJECTIVES
Nobiletin (NOB), a citrus flavonoid, is reported to have beneficial effects on cardiovascular and metabolic health. However, there is limited research investigating the effect of long-term supplementation with low-dose NOB on highcholesterol diet (HCD)-induced hypercholesterolemia and non-obese nonalcoholic fatty liver disease (NAFLD). Therefore, we investigated the influence of NOB on hypercholesterolemia and NAFLD in HCD-fed mice.
SUBJECTS/METHODS
C57BL/6J mice were fed a normal diet (ND) or HCD (35 kcal% fat, 1.25% cholesterol, 0.5% cholic acid) with or without NOB (0.02%) for 20 weeks.
RESULTS
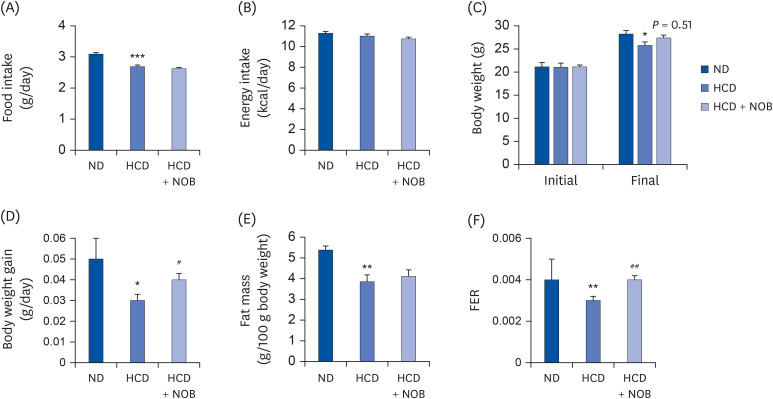
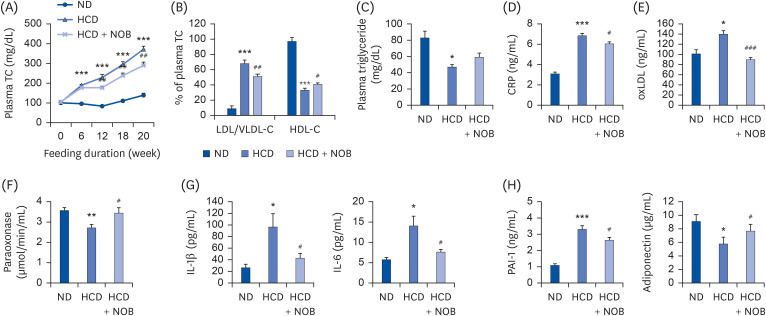
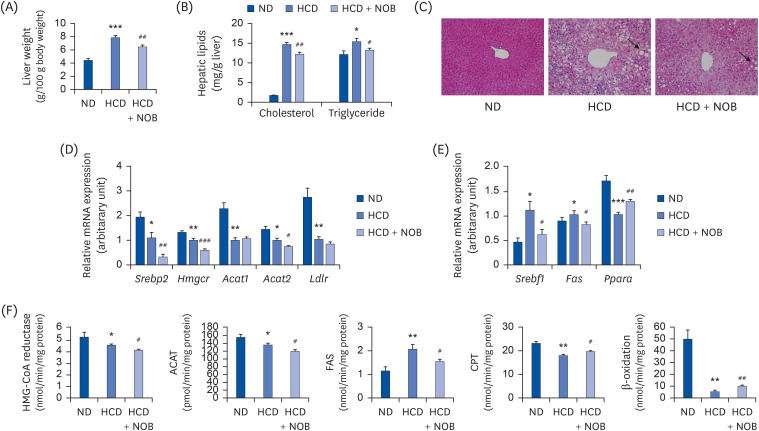
HCD feeding markedly reduced the final body weight compared to ND feeding, with no apparent energy intake differences. NOB supplementation suppressed HCD-induced weight loss without altering energy intake. Moreover, NOB significantly decreased the total cholesterol (TC) levels and the low-density lipoprotein (LDL)/very-LDL-cholesterol to TC ratio, and increased the high-density lipoprotein-cholesterol/TC ratio in plasma, compared to those for HCD feeding alone. The plasma levels of inflammatory and atherosclerosis markers (C-reactive protein, oxidized LDL, interleukin [IL]-1β, IL-6, and plasminogen activator inhibitor-1) were significantly lower, whereas those of anti-atherogenic adiponectin and paraoxonase were higher in the NOB-supplemented group than in the HCD control group. Furthermore, NOB significantly decreased liver weight, hepatic cholesterol and triglyceride contents, and lipid droplet accumulation by inhibiting messenger RNA expression of hepatic genes and activity levels of cholesterol synthesis-, esterification-, and fatty acid synthesis-associated enzymes, concomitantly enhancing fatty acid oxidationrelated gene expression and enzyme activities. Dietary NOB supplementation may protect against hypercholesterolemia and NAFLD via regulation of hepatic lipid metabolism in HCDfed mice; these effects are associated with the amelioration of inflammation and reductions in the levels of atherosclerosis-associated cardiovascular markers.
CONCLUSIONS
The present study suggests that NOB may serve as a potential therapeutic agent for the treatment of HCD-induced hypercholesterolemia and NAFLD.
Figure
Reference
-
1. Yasutake K, Nakamuta M, Shima Y, Ohyama A, Masuda K, Haruta N, Fujino T, Aoyagi Y, Fukuizumi K, Yoshimoto T, et al. Nutritional investigation of non-obese patients with non-alcoholic fatty liver disease: the significance of dietary cholesterol. Scand J Gastroenterol. 2009; 44:471–477. PMID: 19058085.
Article2. Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Fagà E, Silli B, Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003; 37:909–916. PMID: 12668986.
Article3. Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012; 15:665–674. PMID: 22560219.
Article4. Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, Yokoyama M, Honda M, Zen Y, Nakanuma Y, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007; 46:1392–1403. PMID: 17929294.
Article5. Tu LN, Showalter MR, Cajka T, Fan S, Pillai VV, Fiehn O, Selvaraj V. Metabolomic characteristics of cholesterol-induced non-obese nonalcoholic fatty liver disease in mice. Sci Rep. 2017; 7:6120. PMID: 28733574.
Article6. Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005; 54:3541–3546. PMID: 16306373.
Article7. Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007; 13:1579–1584. PMID: 17461452.
Article8. Huang H, Li L, Shi W, Liu H, Yang J, Yuan X, Wu L. The multifunctional effects of nobiletin and its metabolites in vivo and in vitro . Evid Based Complement Alternat Med. 2016; 2016:2918796. PMID: 27761146.9. Yuk T, Kim Y, Yang J, Sung J, Jeong HS, Lee J. Nobiletin inhibits hepatic lipogenesis via activation of AMP-activated protein kinase. Evid Based Complement Alternat Med. 2018; 2018:7420265. PMID: 29552085.
Article10. Cirillo P, Conte S, Cimmino G, Pellegrino G, Ziviello F, Barra G, Sasso FC, Borgia F, De Palma R, Trimarco B. Nobiletin inhibits oxidized-LDL mediated expression of tissue factor in human endothelial cells through inhibition of NF-κB. Biochem Pharmacol. 2017; 128:26–33. PMID: 28017776.
Article11. Mulvihill EE, Assini JM, Lee JK, Allister EM, Sutherland BG, Koppes JB, Sawyez CG, Edwards JY, Telford DE, Charbonneau A, et al. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes. 2011; 60:1446–1457. PMID: 21471511.
Article12. Burke AC, Sutherland BG, Telford DE, Morrow MR, Sawyez CG, Edwards JY, Drangova M, Huff MW. Intervention with citrus flavonoids reverses obesity and improves metabolic syndrome and atherosclerosis in obese Ldlr−/− mice. J Lipid Res. 2018; 59:1714–1728. PMID: 30008441.13. Kim YJ, Choi MS, Woo JT, Jeong MJ, Kim SR, Jung UJ. Long-term dietary supplementation with low-dose nobiletin ameliorates hepatic steatosis, insulin resistance, and inflammation without altering fat mass in diet-induced obesity. Mol Nutr Food Res. 2017; 61:1600889.
Article14. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509. PMID: 13428781.
Article15. Shapiro DJ, Nordstrom JL, Mitschelen JJ, Rodwell VW, Schimke RT. Micro assay for 3-hydroxy-3-methylglutaryl-CoA reductase in rat liver and in L-cell fibroblasts. Biochim Biophys Acta. 1974; 370:369–377. PMID: 4441486.16. Erickson SK, Shrewsbury MA, Brooks C, Meyer DJ. Rat liver acyl-coenzyme A:cholesterol acyltransferase: its regulation in vivo and some of its properties in vitro . J Lipid Res. 1980; 21:930–941. PMID: 7441061.17. Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty-acid synthase from rat liver. Methods Enzymol. 1975; 35:37–44. PMID: 1121291.18. Markwell MA, McGroarty EJ, Bieber LL, Tolbert NE. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973; 248:3426–3432. PMID: 4702872.19. Lazarow PB. Assay of peroxisomal β-oxidation of fatty acids. Methods Enzymol. 1981; 72:315–319. PMID: 7031421.20. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254. PMID: 942051.
Article21. Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991; 286:152–154. PMID: 1650712.
Article22. Paigen B, Mitchell D, Reue K, Morrow A, Lusis AJ, LeBoeuf RC. Ath-1, a gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Proc Natl Acad Sci U S A. 1987; 84:3763–3767. PMID: 3473481.
Article23. Jawień J, Nastałek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol. 2004; 55:503–517. PMID: 15381823.24. Whitman SC, Kurowska EM, Manthey JA, Daugherty A. Nobiletin, a citrus flavonoid isolated from tangerines, selectively inhibits class A scavenger receptor-mediated metabolism of acetylated LDL by mouse macrophages. Atherosclerosis. 2005; 178:25–32. PMID: 15585197.
Article25. Nohara K, Nemkov T, D'Alessandro A, Yoo SH, Chen Z. Coordinate regulation of cholesterol and bile acid metabolism by the clock modifier nobiletin in metabolically challenged old mice. Int J Mol Sci. 2019; 20:4281.
Article26. Morrow NM, Burke AC, Samsoondar JP, Seigel KE, Wang A, Telford DE, Sutherland BG, O'Dwyer C, Steinberg GR, Fullerton MD, et al. The citrus flavonoid nobiletin confers protection from metabolic dysregulation in high-fat-fed mice independent of AMPK. J Lipid Res. 2020; 61:387–402. PMID: 31964763.
Article27. Owens AP, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, Williams JC, Hubbard BK, Dutton JA, Wang J, et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. 2012; 122:558–568.
Article28. Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004; 45:993–1007. PMID: 15060092.29. Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005; 112:651–657. PMID: 16043640.
Article30. Rifai N, Buring JE, Lee IM, Manson JE, Ridker PM. Is C-reactive protein specific for vascular disease in women? Ann Intern Med. 2002; 136:529–533. PMID: 11926788.
Article31. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002; 347:1557–1565. PMID: 12432042.
Article32. Zhang D, Jiang SL, Rzewnicki D, Samols D, Kushner I. The effect of interleukin-1 on C-reactive protein expression in Hep3B cells is exerted at the transcriptional level. Biochem J. 1995; 310:143–148. PMID: 7646436.
Article33. Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000; 148:209–214. PMID: 10657556.
Article34. Weinhold B, Rüther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem J. 1997; 327:425–429. PMID: 9359411.
Article35. Dawson S, Henney A. The status of PAI-1 as a risk factor for arterial and thrombotic disease: a review. Atherosclerosis. 1992; 95:105–117. PMID: 1418086.
Article36. Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012; 165:574–590. PMID: 21457225.
Article37. Baluta MM, Vintila MM. PAI-1 inhibition - another therapeutic option for cardiovascular protection. Maedica (Bucur). 2015; 10:147–152. PMID: 28275409.38. Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, Fogelman AM, Lusis AJ, Young S, Reddy ST. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins: anti-atherogenic role for paraoxonase-2. J Biol Chem. 2006; 281:29491–29500. PMID: 16891303.39. Litvinov D, Mahini H, Garelnabi M. Antioxidant and anti-inflammatory role of paraoxonase 1: implication in arteriosclerosis diseases. N Am J Med Sci. 2012; 4:523–532. PMID: 23181222.40. Trapani L, Segatto M, Pallottini V. Regulation and deregulation of cholesterol homeostasis: the liver as a metabolic “power station”. World J Hepatol. 2012; 4:184–190. PMID: 22761969.
Article41. Parini P, Davis M, Lada AT, Erickson SK, Wright TL, Gustafsson U, Sahlin S, Einarsson C, Eriksson M, Angelin B, et al. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation. 2004; 110:2017–2023. PMID: 15451793.42. Zhang J, Kelley KL, Marshall SM, Davis MA, Wilson MD, Sawyer JK, Farese RV Jr, Brown JM, Rudel LL. Tissue-specific knockouts of ACAT2 reveal that intestinal depletion is sufficient to prevent diet-induced cholesterol accumulation in the liver and blood. J Lipid Res. 2012; 53:1144–1152. PMID: 22460046.43. Meiner VL, Cases S, Myers HM, Sande ER, Bellosta S, Schambelan M, Pitas RE, McGuire J, Herz J, Farese RV Jr. Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc Natl Acad Sci U S A. 1996; 93:14041–14046. PMID: 8943057.
Article44. Sharpe LJ, Brown AJ. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). J Biol Chem. 2013; 288:18707–18715. PMID: 23696639.
Article45. Arguello G, Balboa E, Arrese M, Zanlungo S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim Biophys Acta. 2015; 1852:1765–1778. PMID: 26027904.
Article46. Alger HM, Brown JM, Sawyer JK, Kelley KL, Shah R, Wilson MD, Willingham MC, Rudel LL. Inhibition of acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2) prevents dietary cholesterol-associated steatosis by enhancing hepatic triglyceride mobilization. J Biol Chem. 2010; 285:14267–14274. PMID: 20231283.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-atherosclerotic effects of perilla oil in rabbits fed a high-cholesterol diet
- An Activation of Peroxisome Proliferator-Activated Receptor delta Attenuate Alcoholic Liver Disease and Nonalcoholic Fatty Liver Disease in Rats
- Egg phospholipids exert an inhibitory effect on intestinal cholesterol absorption in mice
- Effects of an aqueous extract of purple sweet potato on nonalcoholic fatty liver in high fat/cholesterol-fed mice
- Efficacy of evogliptin and cenicriviroc against nonalcoholic steatohepatitis in mice: a comparative study