Intest Res.
2021 Jul;19(3):265-274. 10.5217/ir.2020.00045.
Management of Clostridioides difficile infection in patients with inflammatory bowel disease
- Affiliations
-
- 1C. difficile Clinic and Microbial Replacement Therapy Program, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA
- KMID: 2518682
- DOI: http://doi.org/10.5217/ir.2020.00045
Abstract
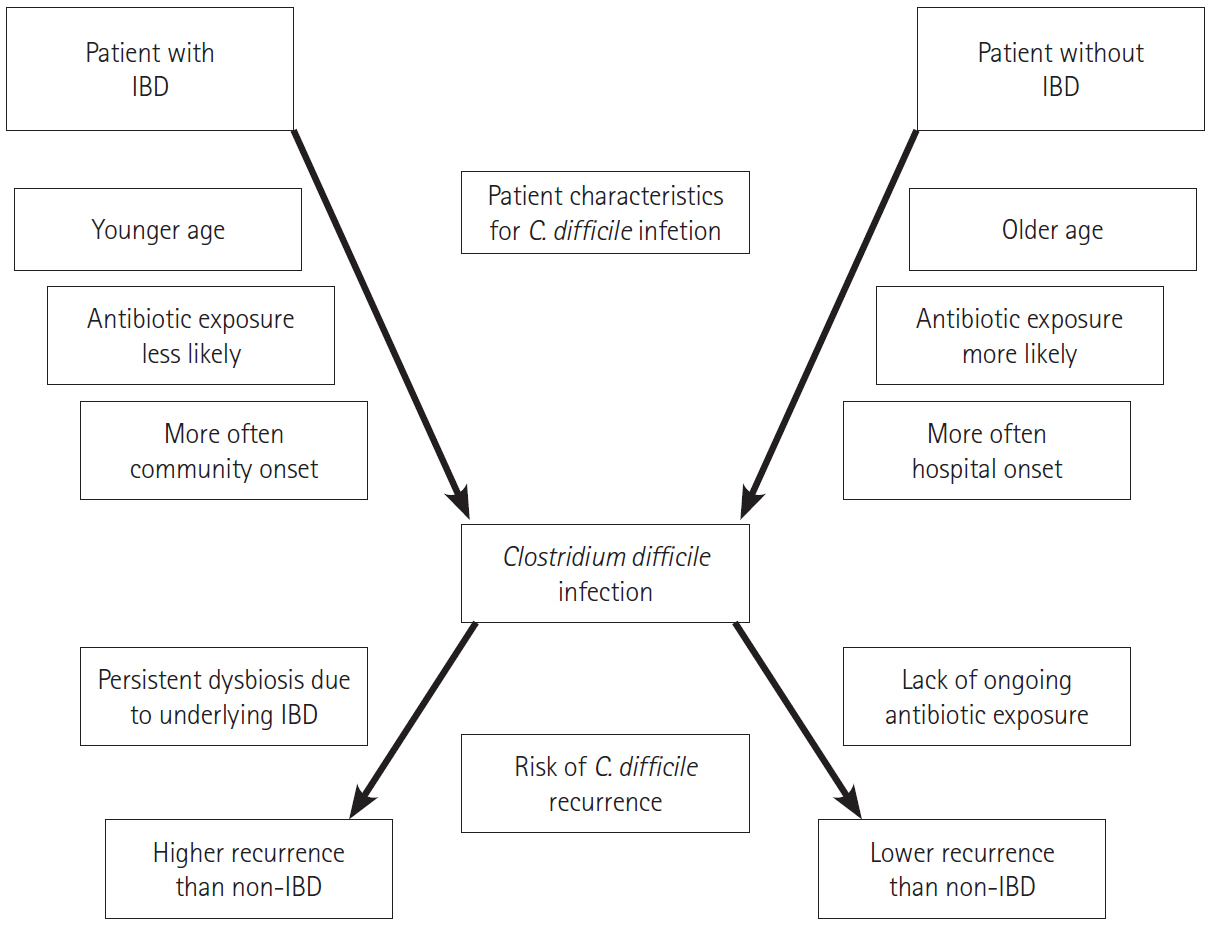
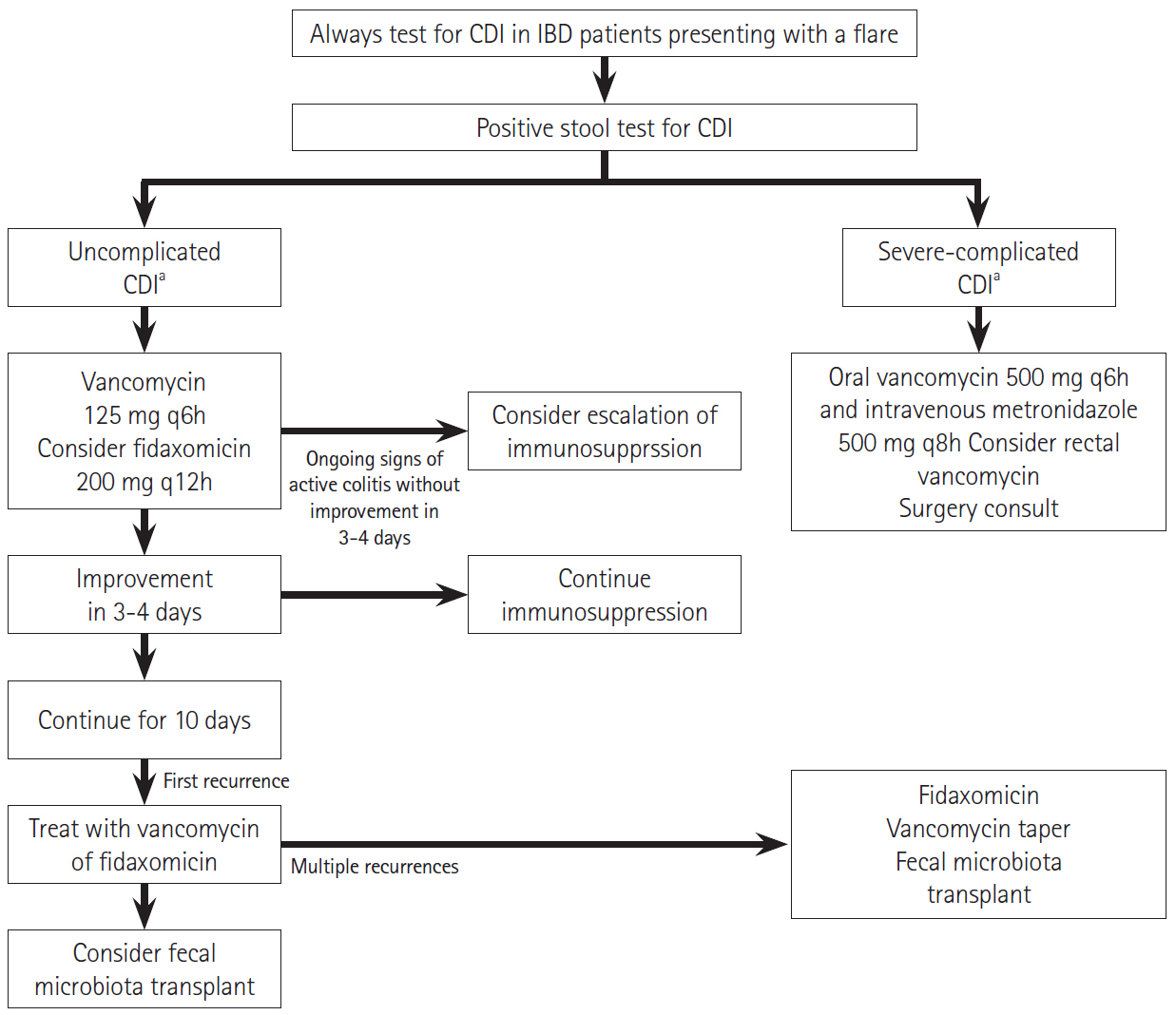
- Inflammatory bowel disease (IBD) is a common diarrheal illness with gastrointestinal and extraintestinal manifestations and complications. The most common infectious complication associated with IBD is Clostridioides difficile infection (CDI). Active IBD predisposes to CDI due to alterations in the gut microbiome. C. difficile is a toxin producing bacterium leading to worsening of underlying IBD, increasing the risk of IBD treatment failure and an increased risk of hospitalization and surgery. Since the symptoms of CDI overlap with those of an IBD flare; it is prudent to recognize that the diagnosis of CDI is challenging and diagnostic tests (nucleic-acid and toxin-based assays) should be interpreted in context of symptoms and test performance. First line treatments for management of CDI in IBD include vancomycin or fidaxomicin. Recurrence prevention strategies should be implemented to mitigate recurrent CDI risk. One needs to monitor IBD disease progression and manage immunosuppression. The risk of recurrent CDI after a primary infection is higher in IBD compared to non-IBD patients. Microbiota restoration therapies are effective to prevent recurrent CDI in IBD patients. This review summarizes the epidemiology, pathophysiology, diagnostic testing, outcomes and management of both CDI and IBD, in CDI complicating IBD.
Figure
Reference
-
1. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015; 12:720–727.
Article2. Franzosa EA, Sirota-Madi A, Avila-Pacheco J, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019; 4:293–305.
Article3. Khanna S, Shin A, Kelly CP. Management of Clostridium difficile infection in inflammatory bowel disease: expert review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017; 15:166–174.
Article4. Hanada Y, Khanna S, Loftus EV Jr, Raffals LE, Pardi DS. NonClostridium difficile bacterial infections are rare in patients with flares of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2018; 16:528–533.
Article5. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015; 373:287–288.6. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018; 66:e1–e48.
Article7. Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020; 382:1320–1330.
Article8. Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012; 107:89–95.
Article9. Bossuyt P, Verhaegen J, van Assche G, Rutgeerts P, Vermeire S. Increasing incidence of Clostridium difficile-associated diarrhea in inflammatory bowel disease. J Crohns Colitis. 2009; 3:4–7.
Article10. Rodemann JF, Dubberke ER, Reske KA, Seo DH, Stone CD. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007; 5:339–344.
Article11. Ramos-Martínez A, Ortiz-Balbuena J, Curto-García I, et al. Risk factors for Clostridium difficile diarrhea in patients with inflammatory bowel disease. Rev Esp Enferm Dig. 2015; 107:4–8.12. Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007; 5:345–351.
Article13. Balram B, Battat R, Al-Khoury A, et al. Risk factors associated with Clostridium difficile infection in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2019; 13:27–38.
Article14. Moens A, Verstockt B, Machiels K, et al. Clostridium difficile infection in inflammatory bowel disease: epidemiology over two decades. Eur J Gastroenterol Hepatol. 2019; 31:668–673.
Article15. Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008; 57:205–210.
Article16. Khanna S, Pardi DS. Clinical implications of antibiotic impact on gastrointestinal microbiota and Clostridium difficile infection. Expert Rev Gastroenterol Hepatol. 2016; 10:1145–1152.
Article17. Clayton EM, Rea MC, Shanahan F, et al. The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission. Am J Gastroenterol. 2009; 104:1162–1169.
Article18. Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014; 146:1489–1499.
Article19. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007; 104:13780–13785.
Article20. Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014; 63:1275–1283.
Article21. Baumgart M, Dogan B, Rishniw M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J. 2007; 1:403–418.
Article22. Nemoto H, Kataoka K, Ishikawa H, et al. Reduced diversity and imbalance of fecal microbiota in patients with ulcerative colitis. Dig Dis Sci. 2012; 57:2955–2964.
Article23. Bajer L, Kverka M, Kostovcik M, et al. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol. 2017; 23:4548–4558.
Article24. Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012; 13:R79.
Article25. Giel JL, Sorg JA, Sonenshein AL, Zhu J. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One. 2010; 5:e8740.
Article26. Allegretti JR, Kearney S, Li N, et al. Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment Pharmacol Ther. 2016; 43:1142–1153.
Article27. Jodorkovsky D, Young Y, Abreu MT. Clinical outcomes of patients with ulcerative colitis and co-existing Clostridium difficile infection. Dig Dis Sci. 2010; 55:415–420.
Article28. Law CC, Tariq R, Khanna S, Murthy S, McCurdy JD. Systematic review with meta-analysis: the impact of Clostridium difficile infection on the short- and long-term risks of colectomy in inflammatory bowel disease. Aliment Pharmacol Ther. 2017; 45:1011–1020.
Article29. Khanna S, Pardi DS. IBD: poor outcomes after Clostridium difficile infection in IBD. Nat Rev Gastroenterol Hepatol. 2012; 9:307–308.
Article30. Tariq R, Law CCY, Khanna S, Murthy S, McCurdy JD. The impact of Clostridium difficile infection on mortality in patients with inflammatory bowel disease: a systematic review and meta-analysis. J Clin Gastroenterol. 2019; 53:127–133.
Article31. Razik R, Rumman A, Bahreini Z, McGeer A, Nguyen GC. Recurrence of Clostridium difficile infection in patients with inflammatory bowel disease: the RECIDIVISM study. Am J Gastroenterol. 2016; 111:1141–1146.
Article32. Saffouri G, Gupta A, Loftus EV Jr, Baddour LM, Pardi DS, Khanna S. The incidence and outcomes from Clostridium difficile infection in hospitalized adults with inflammatory bowel disease. Scand J Gastroenterol. 2017; 52:1240–1247.
Article33. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010; 31:431–455.
Article34. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013; 108:478–498.
Article35. Debast SB, Bauer MP, Kuijper EJ; European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014; 20 Suppl 2:1–26.
Article36. Planche TD, Davies KA, Coen PG, et al. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis. 2013; 13:936–945.
Article37. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015; 175:1792–1801.
Article38. Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000; 342:390–397.
Article39. Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007; 45:992–998.
Article40. Furuya-Kanamori L, Marquess J, Yakob L, et al. Asymptomatic Clostridium difficile colonization: epidemiology and clinical implications. BMC Infect Dis. 2015; 15:516.
Article41. Alasmari F, Seiler SM, Hink T, Burnham CA, Dubberke ER. Prevalence and risk factors for asymptomatic Clostridium difficile carriage. Clin Infect Dis. 2014; 59:216–222.
Article42. Gupta A, Khanna S. Repeat Clostridium difficile testing. JAMA. 2016; 316:2422–2423.43. Gupta A, Cifu AS, Khanna S. Diagnosis and treatment of Clostridium difficile infection. JAMA. 2018; 320:1031–1032.
Article44. Gupta A, Wash C, Wu Y, Sorrentino D, Nguyen VQ. Diagnostic modality of Clostridioides difficile infection predicts treatment response and outcomes in inflammatory bowel disease. Dig Dis Sci. 2021; 66:547–553.
Article45. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014; 59:345–354.
Article46. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011; 364:422–431.
Article47. Guery B, Menichetti F, Anttila VJ, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis. 2018; 18:296–307.
Article48. Horton HA, Dezfoli S, Berel D, et al. Antibiotics for treatment of Clostridium difficile infection in hospitalized patients with inflammatory bowel disease. Antimicrob Agents Chemother. 2014; 58:5054–5059.
Article49. Lei DK, Ollech JE, Andersen M, et al. Long-duration oral vancomycin to treat Clostridioides difficile in patients with inflammatory bowel disease is associated with a low rate of recurrence. Am J Gastroenterol. 2019; 114:1904–1908.
Article50. Spiceland CM, Saffouri G, Pardi D, Khanna S. Mo1656 Outcomes of fidaxomicin treatment of Clostridium difficile infection. Gastroenterology. 2016; 150(4 Suppl 1):S744.
Article51. Bartsch SM, Umscheid CA, Fishman N, Lee BY. Is fidaxomicin worth the cost? An economic analysis. Clin Infect Dis. 2013; 57:555–561.
Article52. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017; 376:305–317.
Article53. Kelly CP, Wilcox MH, Glerup H, et al. Bezlotoxumab for Clostridium difficile infection complicating inflammatory bowel disease. Gastroenterology. 2018; 155:1270–1271.
Article54. Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014; 109:1065–1071.
Article55. Nanki K, Mizuno S, Matsuoka K, et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection in a patient with ulcerative colitis. Intest Res. 2018; 16:142–146.
Article56. Tariq R, Disbrow MB, Dibaise JK, et al. Efficacy of fecal microbiota transplantation for recurrent C. difficile infection in inflammatory bowel disease. Inflamm Bowel Dis. 2020; 26:1415–1420.
Article57. Khoruts A, Rank KM, Newman KM, et al. Inflammatory bowel disease affects the outcome of fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2016; 14:1433–1438.
Article58. Fischer M, Kao D, Kelly C, et al. Fecal microbiota transplantation is safe and efficacious for recurrent or refractory Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016; 22:2402–2409.
Article59. You JHS, Jiang X, Lee WH, Chan PKS, Ng SC. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection in patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2020; 35:1515–1523.
Article60. Khanna S, Vazquez-Baeza Y, González A, et al. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome. 2017; 5:55.
Article61. Knox NC, Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiome as a target for IBD treatment: are we there yet? Curr Treat Options Gastroenterol. 2019; 17:115–126.
Article62. Bak SH, Choi HH, Lee J, et al. Fecal microbiota transplantation for refractory Crohn’s disease. Intest Res. 2017; 15:244–248.
Article63. DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019; 381:2043–2050.
Article64. Ben-Horin S, Margalit M, Bossuyt P, et al. Combination immunomodulator and antibiotic treatment in patients with inflammatory bowel disease and Clostridium difficile infection. Clin Gastroenterol Hepatol. 2009; 7:981–987.
Article65. Ananthakrishnan AN, Guzman-perez R, Gainer V, et al. Predictors of severe outcomes associated with Clostridium difficile infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012; 35:789–795.
Article66. Lukin DJ, Lawlor G, Hudesman DP, et al. Escalation of immunosuppressive therapy for inflammatory bowel disease is not associated with adverse outcomes after infection with Clostridium difficile. Inflamm Bowel Dis. 2019; 25:775–781.
Article67. Solanky D, Pardi DS, Loftus EV, Khanna S. Colon surgery risk with corticosteroids versus immunomodulators or biologics in inflammatory bowel disease patients with Clostridium difficile infection. Inflamm Bowel Dis. 2019; 25:610–619.
Article68. Bar-Yoseph H, Daoud H, Ben Hur D, Chowers Y, Waterman M. Does early corticosteroid therapy affect prognosis in IBD patients hospitalized with Clostridioides difficile infection? Int J Colorectal Dis. 2020; 35:513–519.
Article69. Yanai H, Nguyen GC, Yun L, et al. Practice of gastroenterologists in treating flaring inflammatory bowel disease patients with Clostridium difficile: antibiotics alone or combined antibiotics/immunomodulators? Inflamm Bowel Dis. 2011; 17:1540–1546.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clostridioides Infection in Patients with Inflammatory Bowel Disease
- Durability and outcomes of fecal microbiota transplantation for recurrent Clostridioides difficile infection in patients with moderate to severe inflammatory bowel disease
- Left-sided Ulcerative Colitis Reactivated and Aggravated during Clostridium difficile Infection
- Which is the Preferred Regimen for Non-Severe Clostridioides difficile Infection in Korea, Vancomycin or Metronidazole?
- Fecal Microbiota Transplantation beyond Clostridioides Difficile Infection