Cancer Res Treat.
2021 Jul;53(3):819-828. 10.4143/crt.2020.1013.
Enhanced Efficacy of Combined Therapy with Checkpoint Kinase 1 Inhibitor and Rucaparib via Regulation of Rad51 Expression in BRCA Wild-Type Epithelial Ovarian Cancer Cells
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Obstetrics and Gynecology, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
- 3Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2518406
- DOI: http://doi.org/10.4143/crt.2020.1013
Abstract
- Purpose
This study aimed to evaluate anticancer effects of combination treatment with poly(ADP-ribose) polymerase (PARP) and checkpoint kinase 1 (Chk1) inhibitors in BRCA wild-type ovarian cancer. PARP inhibitors can function as DNA-damaging agents in BRCA wild-type cancer, even if clinical activity is limited. Most epithelial ovarian cancers are characterized by a TP53 mutation causing dysfunction at the G1/S checkpoint, which makes tumor cells highly dependent on Chk1-mediated G/M phase cell-cycle arrest for DNA repair.
Materials and Methods
We investigated the anticancer effects of combination treatment with prexasertib (LY2606368), a selective ATP competitive small molecule inhibitor of Chk1 and Chk2, and rucaparib, a PARP inhibitor, in BRCA wild-type ovarian cancer cell lines (OVCAR3 and SKOV3).
Results
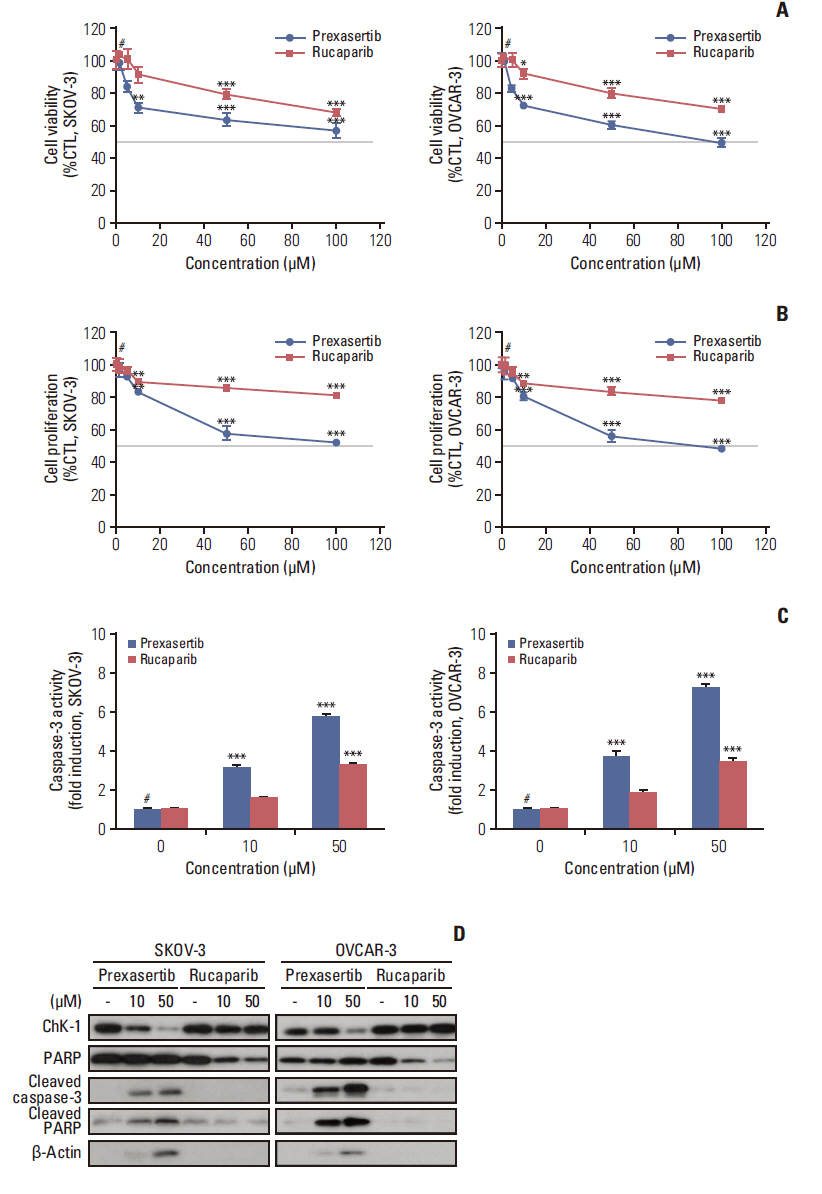
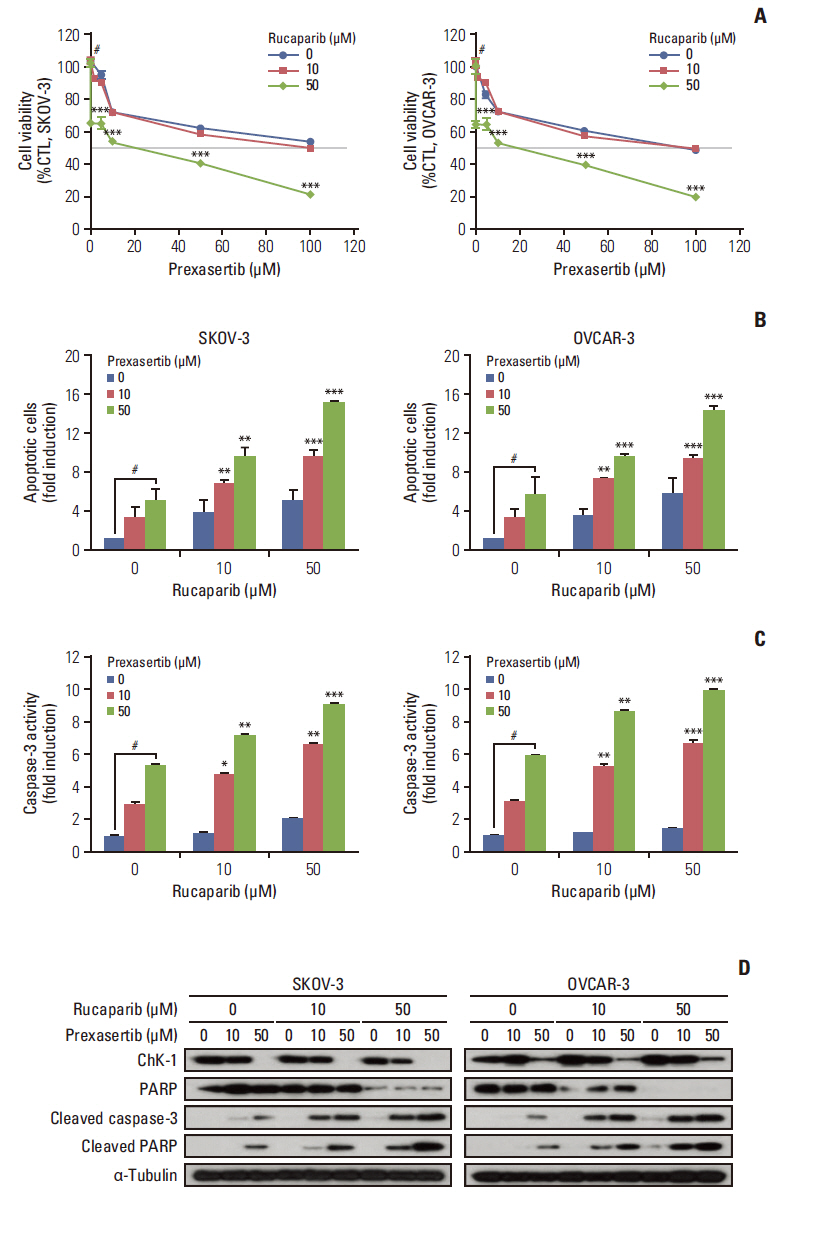
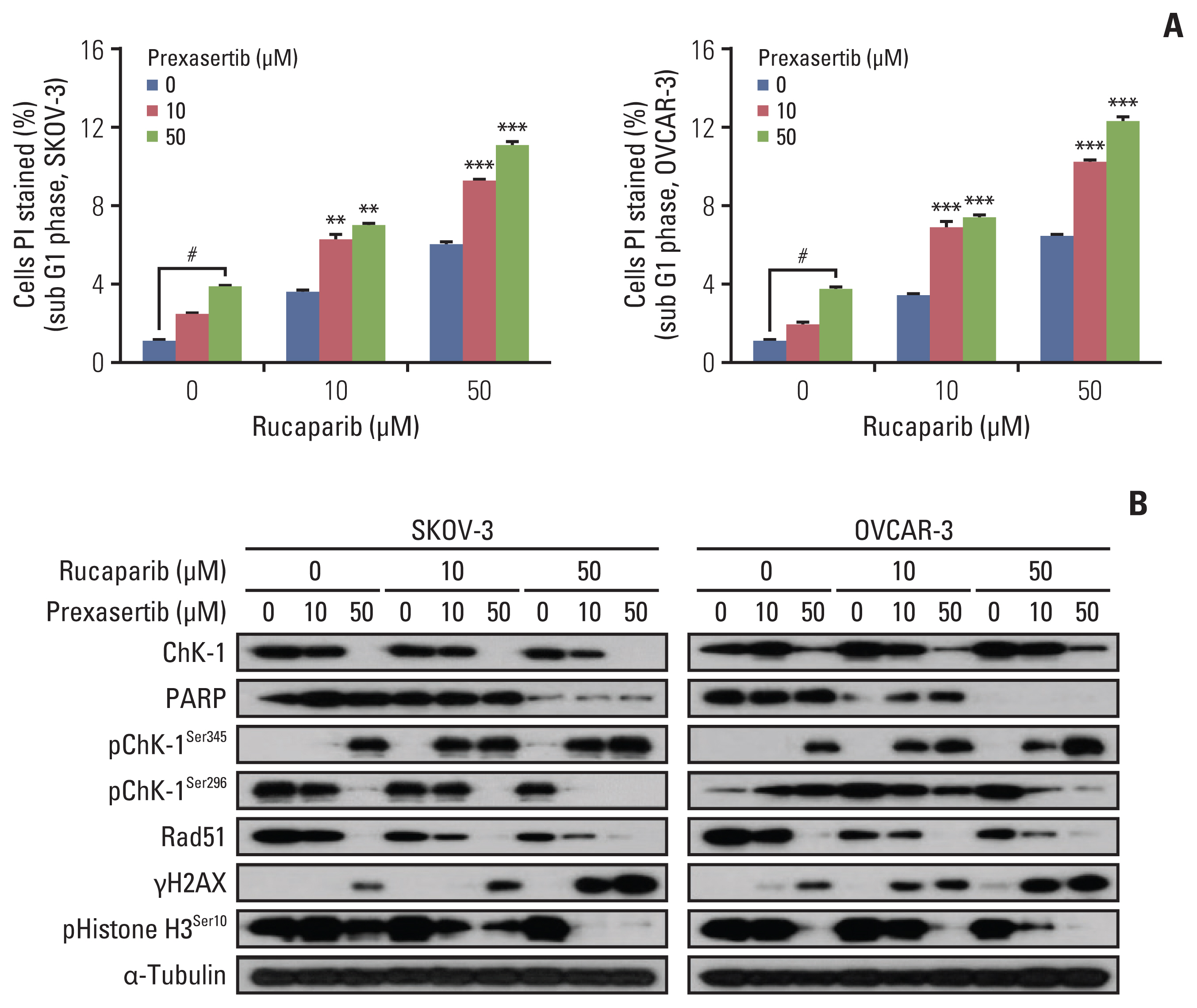
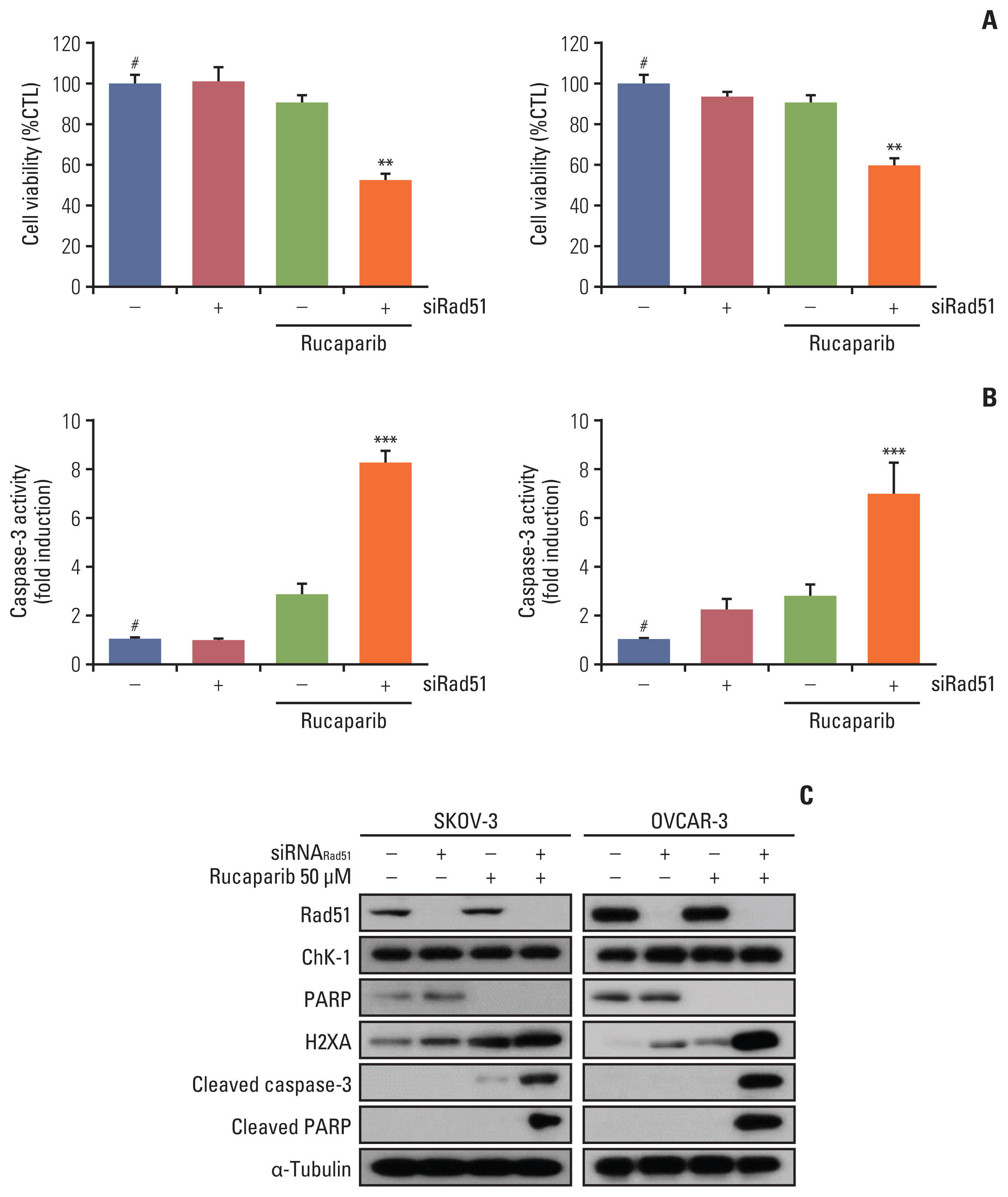
We found that combined treatment significantly decreased cell viability in all cell lines and induced greater DNA damage and apoptosis than in the control and/or using monotherapies. Moreover, we found that prexasertib significantly inhibited homologous recombination–mediated DNA repair and thus showed a marked anticancer effect in combination treatment with rucaparib. The anticancer mechanism of prexasertib and rucaparib was considered to be caused by an impaired G2/M checkpoint due to prexasertib treatment, which forced mitotic catastrophe in the presence of rucaparib.
Conclusion
Our results suggest a novel effective therapeutic strategy for BRCA wild-type epithelial ovarian cancer using a combination of Chk1 and PARP inhibitors.
Keyword
Figure
Reference
-
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Jung KW, Won YJ, Hong S, Kong HJ, Lee ES. Prediction of cancer incidence and mortality in Korea, 2020. Cancer Res Treat. 2020; 52:351–8.
Article3. Tian H, Gao Z, Li H, Zhang B, Wang G, Zhang Q, et al. DNA damage response: a double-edged sword in cancer prevention and cancer therapy. Cancer Lett. 2015; 358:8–16.4. Kim H, George E, Ragland R, Rafail S, Zhang R, Krepler C, et al. Targeting the ATR/CHK1 axis with PARP inhibition results in tumor regression in BRCA-mutant ovarian cancer models. Clin Cancer Res. 2017; 23:3097–108.
Article5. Reaper PM, Griffiths MR, Long JM, Charrier JD, Maccormick S, Charlton PA, et al. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat Chem Biol. 2011; 7:428–30.
Article6. Chen Z, Xiao Z, Gu WZ, Xue J, Bui MH, Kovar P, et al. Selective Chk1 inhibitors differentially sensitize p53-deficient cancer cells to cancer therapeutics. Int J Cancer. 2006; 119:2784–94.
Article7. Karnitz LM, Zou L. Molecular pathways: targeting ATR in cancer therapy. Clin Cancer Res. 2015; 21:4780–5.
Article8. Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017; 355:1152–8.
Article9. Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011; 5:387–93.
Article10. Meehan RS, Chen AP. New treatment option for ovarian cancer: PARP inhibitors. Gynecol Oncol Res Pract. 2016; 3:3.
Article11. Murai J. Targeting DNA repair and replication stress in the treatment of ovarian cancer. Int J Clin Oncol. 2017; 22:619–28.
Article12. Klein HL. The consequences of Rad51 overexpression for normal and tumor cells. DNA Repair (Amst). 2008; 7:686–93.
Article13. Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol. 2005; 7:195–201.
Article14. Bahassi EM, Ovesen JL, Riesenberg AL, Bernstein WZ, Hasty PE, Stambrook PJ. The checkpoint kinases Chk1 and Chk2 regulate the functional associations between hBRCA2 and Rad51 in response to DNA damage. Oncogene. 2008; 27:3977–85.
Article15. Matulonis UA, Penson RT, Domchek SM, Kaufman B, Shapira-Frommer R, Audeh MW, et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: a multistudy analysis of response rates and safety. Ann Oncol. 2016; 27:1013–9.
Article16. Loseva O, Jemth AS, Bryant HE, Schuler H, Lehtio L, Karlberg T, et al. PARP-3 is a mono-ADP-ribosylase that activates PARP-1 in the absence of DNA. J Biol Chem. 2010; 285:8054–60.
Article17. LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019; 20:e15–28.
Article18. Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017; 18:75–87.
Article19. Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012; 107:1776–82.
Article20. Krajewska M, Fehrmann RS, Schoonen PM, Labib S, de Vries EG, Franke L, et al. ATR inhibition preferentially targets homologous recombination-deficient tumor cells. Oncogene. 2015; 34:3474–81.
Article21. Booth L, Roberts J, Poklepovic A, Dent P. The CHK1 inhibitor SRA737 synergizes with PARP1 inhibitors to kill carcinoma cells. Cancer Biol Ther. 2018; 19:786–96.
Article22. Yin Y, Shen Q, Zhang P, Tao R, Chang W, Li R, et al. Chk1 inhibition potentiates the therapeutic efficacy of PARP inhibitor BMN673 in gastric cancer. Am J Cancer Res. 2017; 7:473–83.23. Brill E, Yokoyama T, Nair J, Yu M, Ahn YR, Lee JM. Prexasertib, a cell cycle checkpoint kinases 1 and 2 inhibitor, increases in vitro toxicity of PARP inhibition by preventing Rad51 foci formation in BRCA wild type high-grade serous ovarian cancer. Oncotarget. 2017; 8:111026–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targeted therapy and immunotherapy in ovarian cancer
- Targeted therapy of ovarian cancer including immune check point inhibitor
- A single-arm phase II study of olaparib maintenance with pembrolizumab and bevacizumab in BRCA non-mutated patients with platinum-sensitive recurrent ovarian cancer (OPEB-01)
- RAD51/geminin/γH2AX immunohistochemical expression predicts platinum-based chemotherapy response in ovarian high-grade serous carcinoma
- Prevalence and oncologic outcomes of BRCA1/2 mutation and variant of unknown significance in epithelial ovarian carcinoma patients in Korea