Cancer Res Treat.
2021 Jul;53(3):703-713. 10.4143/crt.2020.805.
Optimal Maintenance Strategy for First-Line Oxaliplatin-Containing Therapy with or without Bevacizumab in Patients with Metastatic Colorectal Cancer: A Meta-Analysis
- Affiliations
-
- 1Department of Gastroenterology, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- 2Department of Biostatistics, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- KMID: 2518394
- DOI: http://doi.org/10.4143/crt.2020.805
Abstract
- Purpose
Maintenance therapy after oxaliplatin withdrawal is useful in patients with metastatic colorectal cancer (mCRC). This study aimed to investigate the timing of discontinuation or reintroduction of oxaliplatin and the optimal maintenance therapy regimen for survival.
Materials and Methods
PubMed and conference abstracts were searched to select phase II and III trials of first-line oxaliplatin-containing therapy with or without bevacizumab using maintenance therapy for mCRC. Correlations of median overall survival (OS) with induction therapy regimens, induction therapy duration, maintenance therapy regimens (fluoropyrimidine plus bevacizumab [FP+Bev], FP/Bev alone, and no treatment), and oxaliplatin reintroduction were investigated using correlation and weighted multivariate regression analyses.
Results
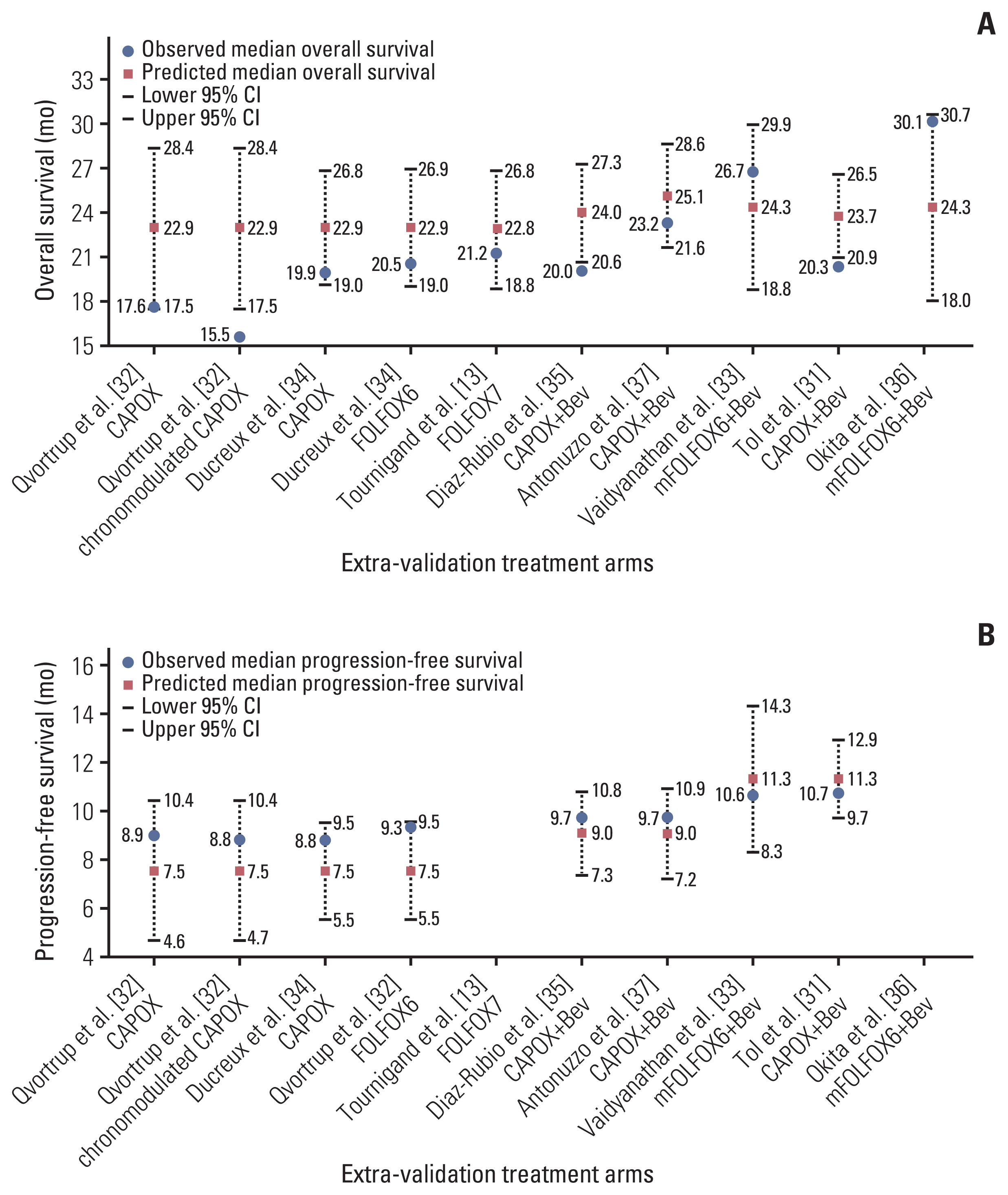
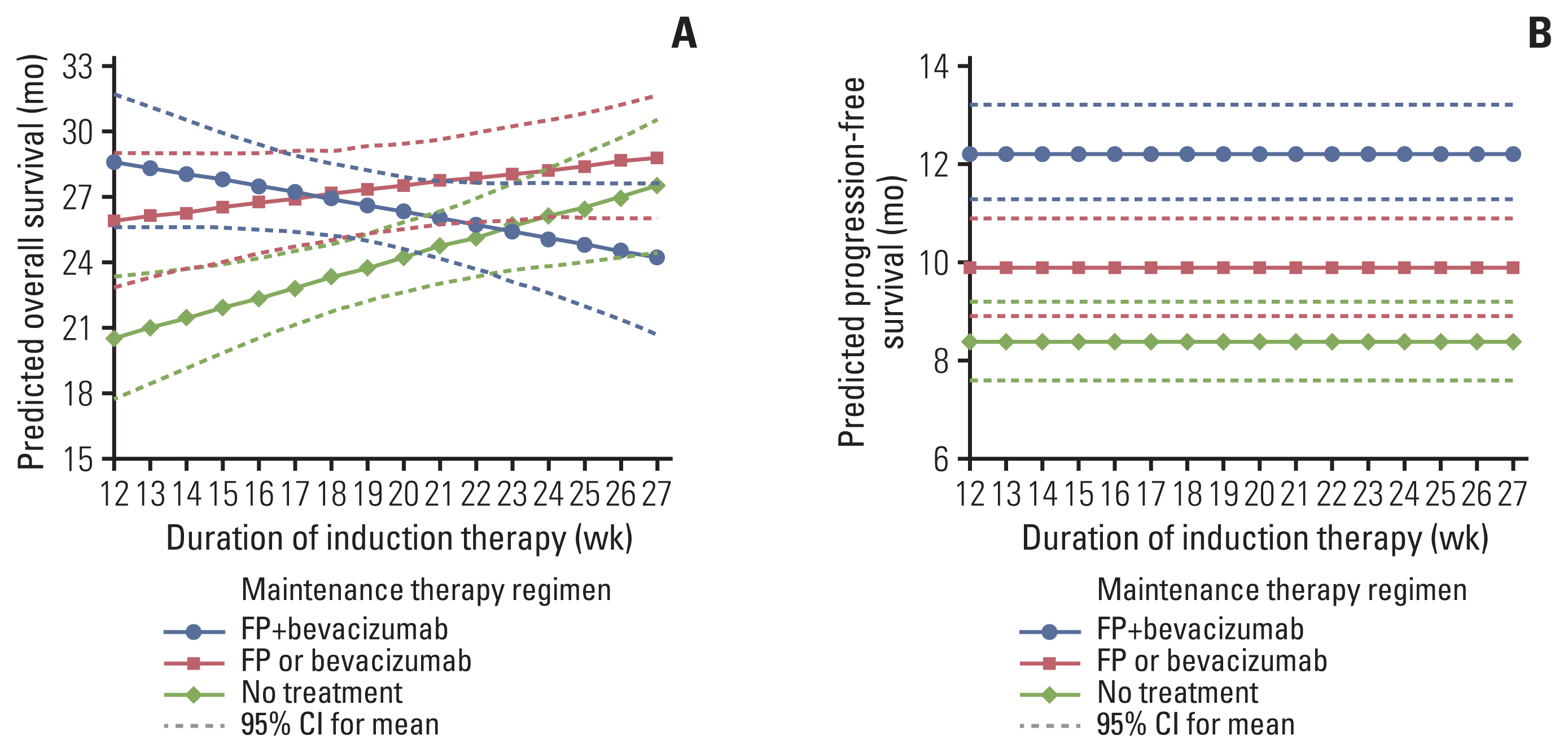
Twenty-two treatment arms were analyzed, including 2,581 patients. The maintenance therapy regimen FP+Bev showed the strongest correlation with a prolonged OS (Spearman’s partial correlation coefficient=0.42), and the other three variables correlated weakly with the OS. The maintenance therapy regimen significantly interacted with the induction chemotherapy duration (p=0.019). The predicted OS for FP+Bev crossed the lines of FP/Bev alone at 18 weeks of induction therapy, and of no treatment at 23 weeks. The corresponding OS at 12 and 27 weeks of induction therapies were 28.6 and 24.2 months for FP+Bev, 25.9 and 28.8 months for FP/Bev alone, and 20.5 and 27.5 months for no treatment.
Conclusion
The optimal maintenance therapy regimen for the OS is a continuous induction therapy as long as possible followed by FP/Bev alone and switching to FP+Bev within approximately 4 months if induction therapy is discontinued.
Keyword
Figure
Reference
-
References
1. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938–47.
Article2. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006; 24:3347–53.
Article3. Porschen R, Arkenau HT, Kubicka S, Greil R, Seufferlein T, Freier W, et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol. 2007; 25:4217–23.
Article4. Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004; 22:229–37.
Article5. Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010; 28:4697–705.
Article6. Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008; 26:2013–9.
Article7. Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized cinical trial. JAMA. 2017; 317:2392–401.8. Grothey A, Nikcevich DA, Sloan JA, Kugler JW, Silberstein PT, Dentchev T, et al. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol. 2011; 29:421–7.
Article9. Hochster HS, Grothey A, Hart L, Rowland K, Ansari R, Alberts S, et al. Improved time to treatment failure with an intermittent oxaliplatin strategy: results of CONcePT. Ann Oncol. 2014; 25:1172–8.
Article10. Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn KA, Atherton P, et al. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol. 2014; 32:997–1005.
Article11. Oki E, Emi Y, Kojima H, Higashijima J, Kato T, Miyake Y, et al. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol. 2015; 20:767–75.
Article12. Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013; 309:1359–67.13. Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol. 2006; 24:394–400.
Article14. Berry SR, Cosby R, Asmis T, Chan K, Hammad N, Krzyzanowska MK, et al. Continuous versus intermittent chemotherapy strategies in metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2015; 26:477–85.
Article15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009; 6:e1000100.
Article16. Andre T, Tournigand C, Mineur L, Fellague-Chebra R, Flesch M, Mabro M, et al. Phase II study of an optimized 5-fluorouracil-oxaliplatin strategy (OPTIMOX2) with celecoxib in metastatic colorectal cancer: a GERCOR study. Ann Oncol. 2007; 18:77–81.17. Scalamogna R, Brugnatelli S, Tinelli C, Sagrada P, Gattoni E, Tronconi MC, et al. UFT as maintenance therapy in patients with advanced colorectal cancer responsive to the FOLFOX4 regimen. Oncology. 2007; 72:267–73.
Article18. Chibaudel B, Maindrault-Goebel F, Lledo G, Mineur L, Andre T, Bennamoun M, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009; 27:5727–33.
Article19. Comella P, Massidda B, Filippelli G, Farris A, Natale D, Barberis G, et al. Randomised trial comparing biweekly oxaliplatin plus oral capecitabine versus oxaliplatin plus i.v. bolus fluorouracil/leucovorin in metastatic colorectal cancer patients: results of the Southern Italy Cooperative Oncology study 0401. J Cancer Res Clin Oncol. 2009; 135:217–26.
Article20. Waddell T, Gollins S, Soe W, Valle J, Allen J, Bentley D, et al. Phase II study of short-course capecitabine plus oxaliplatin (XELOX) followed by maintenance capecitabine in advanced colorectal cancer: XelQuali study. Cancer Chemother Pharmacol. 2011; 67:1111–7.
Article21. Tezuka T, Hamada C, Ishida H, Ooshiro M, Matsuoka H, Kawasaki S, et al. Phase II clinical study of modified FOLFOX7 (intermittent oxaliplatin administration) plus bevacizumab in patients with unresectable metastatic colorectal cancer-CRAFT study. Invest New Drugs. 2013; 31:1321–9.
Article22. Yalcin S, Uslu R, Dane F, Yilmaz U, Zengin N, Buyukunal E, et al. Bevacizumab+capecitabine as maintenance therapy after initial bevacizumab+XELOX treatment in previously untreated patients with metastatic colorectal cancer: phase III ‘Stop and Go’ study results: a Turkish Oncology Group Trial. Oncology. 2013; 85:328–35.23. Hong YS, Lee SS, Kim KP, Lee JL, Kang YK, Shin SJ, et al. A phase II study of bevacizumab, oxaliplatin, and capecitabine in patients with previously untreated metastatic colorectal cancer: a prospective, multicenter trial of the Korean Cancer Study Group. Am J Clin Oncol. 2014; 37:19–23.24. Kim ST, Hong YS, Lim HY, Lee J, Kim TW, Kim KP, et al. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for the first-line treatment of patients with metastatic colorectal cancer: updated results from a phase 3 trial. BMC Cancer. 2014; 14:883.
Article25. Hegewisch-Becker S, Graeven U, Lerchenmuller CA, Killing B, Depenbusch R, Steffens CC, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015; 16:1355–69.
Article26. Nakayama N, Sato A, Tanaka S, Shimada K, Konishi K, Sasaki E, et al. A phase II study of bevacizumab with modified OPTIMOX1 as first-line therapy for metastatic colorectal cancer: the TCOG-GI 0802 study. Invest New Drugs. 2015; 33:954–62.
Article27. Simkens LH, van Tinteren H, May A, ten Tije AJ, Creemers GJ, Loosveld OJ, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015; 385:1843–52.
Article28. Luo HY, Li YH, Wang W, Wang ZQ, Yuan X, Ma D, et al. Single-agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer: randomized clinical trial of efficacy and safety. Ann Oncol. 2016; 27:1074–81.
Article29. Nakayama G, Ishigure K, Yokoyama H, Uehara K, Kojima H, Ishiyama A, et al. The efficacy and safety of CapeOX plus bevacizumab therapy followed by capecitabine plus bevacizumab maintenance therapy in patients with metastatic colorectal cancer: a multi-center, single-arm, phase II study (CCOG-0902). BMC Cancer. 2017; 17:243.
Article30. Kosugi C, Koda K, Denda T, Ishibashi K, Ishida H, Seike K, et al. VOICE trial: Final results from multicenter phase II study of assessment of clinical efficiency and safety in capecitabine plus intermittent oxaliplatin together with bevacizumab as first-line therapy for patients with advanced colorectal cancer. J Clin Oncol. 2018; 36(4 Suppl):740.31. Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009; 360:563–72.
Article32. Qvortrup C, Jensen BV, Fokstuen T, Nielsen SE, Keldsen N, Glimelius B, et al. A randomized study comparing short-time infusion of oxaliplatin in combination with capecitabine XELOX(30) and chronomodulated XELOX(30) as first-line therapy in patients with advanced colorectal cancer. Ann Oncol. 2010; 21:87–91.
Article33. Vaidyanathan G, Groman A, Wilding G, Fakih MG. Stop and go FOLFOX plus bevacizumab chemotherapy in the first-line treatment of metastatic colorectal cancer. Oncology. 2010; 79:67–71.
Article34. Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer. 2011; 128:682–90.
Article35. Diaz-Rubio E, Gomez-Espana A, Massuti B, Sastre J, Abad A, Valladares M, et al. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist. 2012; 17:15–25.36. Okita NT, Esaki T, Baba E, Sakai D, Tokunaga S, Takiuchi H, et al. A multicenter phase II study of the stop-and-go modified FOLFOX6 with bevacizumab for first-line treatment of patients with metastatic colorectal cancer. Invest New Drugs. 2012; 30:2026–31.
Article37. Antonuzzo L, Giommoni E, Pastorelli D, Latiano T, Pavese I, Azzarello D, et al. Bevacizumab plus XELOX as first-line treatment of metastatic colorectal cancer: The OBELIX study. World J Gastroenterol. 2015; 21:7281–8.
Article38. Grothey A. Clinical management of oxaliplatin-associated neurotoxicity. Clin Colorectal Cancer. 2005; 5(Suppl 1):S38–46.
Article39. Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer. 2014; 22:1999–2007.
Article40. de Gramont A, Buyse M, Abrahantes JC, Burzykowski T, Quinaux E, Cervantes A, et al. Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J Clin Oncol. 2007; 25:3224–9.
Article41. Chibaudel B, Bonnetain F, Shi Q, Buyse M, Tournigand C, Sargent DJ, et al. Alternative end points to evaluate a therapeutic strategy in advanced colorectal cancer: evaluation of progression-free survival, duration of disease control, and time to failure of strategy: an Aide et Recherche en Cancerologie Digestive Group Study. J Clin Oncol. 2011; 29:4199–204.42. Owonikoko TK, Ramalingam SS, Belani CP. Maintenance therapy for advanced non-small cell lung cancer: current status, controversies, and emerging consensus. Clin Cancer Res. 2010; 16:2496–504.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retrospective analysis on the clinical efficacy of bevacizumab combined with FOLFOX4 in the first line treatment of metastatic colorectal cancer
- Drug-induced immune-mediated thrombocytopenia due to bevacizumab-FOLFOX therapy: a case report
- Imrpoving Outcomes with Chemotherapy in Colorectal Cancer: Current Options, Current Evidence
- New Trend in Chemotherapy for Colorectal Cancer
- Chemotherapy for Colorectal Cancer