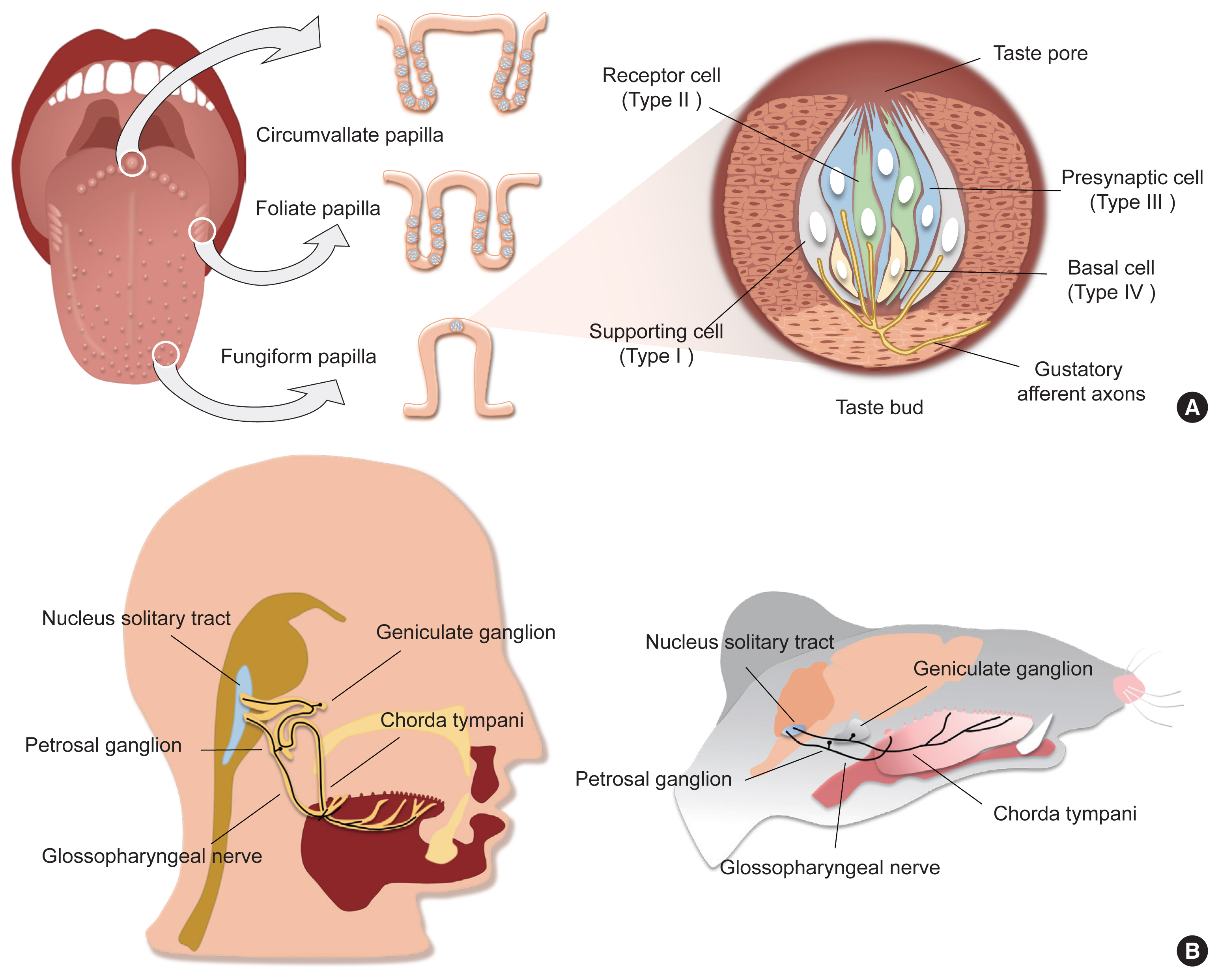
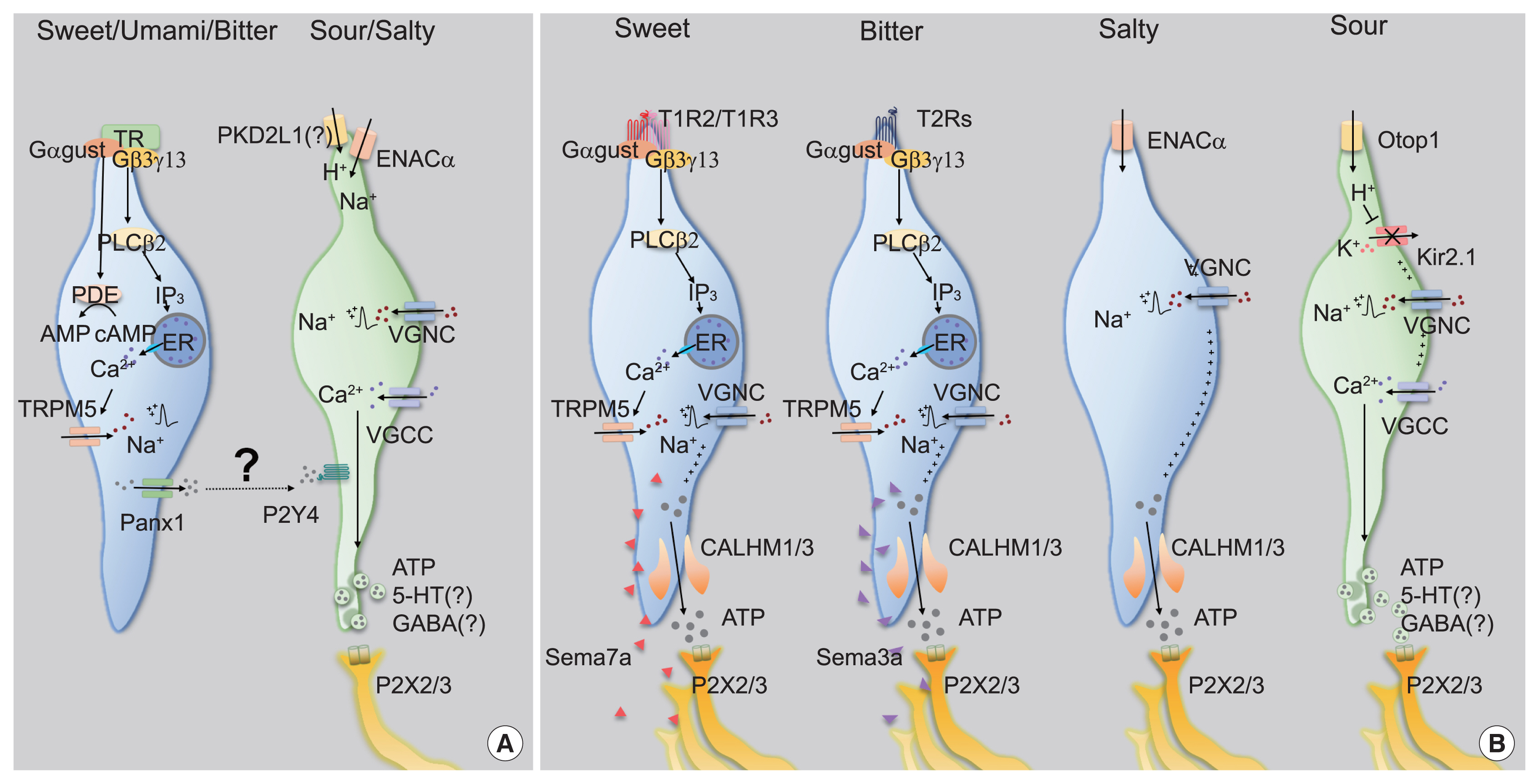
Endocrinol Metab.
2021 Jun;36(3):469-477. 10.3803/EnM.2021.302.
Recent Advances in Understanding Peripheral Taste Decoding I: 2010 to 2020
- Affiliations
-
- 1BK21 Graduate Program, Department of Biomedical Sciences, Korea University College of Medicine, Seoul, Korea
- 2Department of Pharmacology, Korea University College of Medicine, Seoul, Korea
- 3Department of Biochemistry and Molecular Biology, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Oral Biology, BK21 FOUR Project, Yonsei University College of Dentistry, Seoul, Korea
- KMID: 2517636
- DOI: http://doi.org/10.3803/EnM.2021.302
Abstract
- Taste sensation is the gatekeeper for direct decisions on feeding behavior and evaluating the quality of food. Nutritious and beneficial substances such as sugars and amino acids are represented by sweet and umami tastes, respectively, whereas noxious substances and toxins by bitter or sour tastes. Essential electrolytes including Na+ and other ions are recognized by the salty taste. Gustatory information is initially generated by taste buds in the oral cavity, projected into the central nervous system, and finally processed to provide input signals for food recognition, regulation of metabolism and physiology, and higher-order brain functions such as learning and memory, emotion, and reward. Therefore, understanding the peripheral taste system is fundamental for the development of technologies to regulate the endocrine system and improve whole-body metabolism. In this review article, we introduce previous widely-accepted views on the physiology and genetics of peripheral taste cells and primary gustatory neurons, and discuss key findings from the past decade that have raised novel questions or solved previously raised questions.
Keyword
Figure
Reference
-
1. Farbman AI. Fine structure of the taste bud. J Ultrastruct Res. 1965; 12:328–50.
Article2. Kinnamon JC, Sherman TA, Roper SD. Ultrastructure of mouse vallate taste buds: III. Patterns of synaptic connectivity. J Comp Neurol. 1988; 270:1–10. 56–7.
Article3. Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001; 106:381–90.
Article4. Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, et al. An amino-acid taste receptor. Nature. 2002; 416:199–202.
Article5. Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, et al. T2Rs function as bitter taste receptors. Cell. 2000; 100:703–11.
Article6. Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003; 115:255–66.
Article7. McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992; 357:563–9.
Article8. Ruiz-Avila L, McLaughlin SK, Wildman D, McKinnon PJ, Robichon A, Spickofsky N, et al. Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature. 1995; 376:80–5.
Article9. Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003; 112:293–301.10. Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005; 434:225–9.
Article11. Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, et al. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999; 2:1055–62.
Article12. Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, et al. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2007; 282:37225–31.
Article13. Huang YA, Roper SD. Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol. 2010; 588(Pt 13):2343–50.14. Dutta Banik D, Martin LE, Freichel M, Torregrossa AM, Medler KF. TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc Natl Acad Sci U S A. 2018; 115:E772–81.
Article15. Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004; 468:311–21.
Article16. Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007; 104:6436–41.
Article17. Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008; 28:13088–93.
Article18. Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, et al. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005; 25:843–7.
Article19. Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005; 310:1495–9.
Article20. Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013; 495:223–6.
Article21. Ma Z, Taruno A, Ohmoto M, Jyotaki M, Lim JC, Miyazaki H, et al. CALHM3 is essential for rapid ion channel-mediated purinergic neurotransmission of GPCR-mediated tastes. Neuron. 2018; 98:547–61.
Article22. Park S, Williams KW, Liu C, Sohn JW. A neural basis for tonic suppression of sodium appetite. Nat Neurosci. 2020; 23:423–32.
Article23. Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010; 464:297–301.
Article24. Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. High salt recruits aversive taste pathways. Nature. 2013; 494:472–5.
Article25. Garty H. Molecular properties of epithelial, amiloride-blockable Na+ channels. FASEB J. 1994; 8:522–8.26. Lossow K, Hermans-Borgmeyer I, Meyerhof W, Behrens M. Segregated expression of ENaC subunits in taste cells. Chem Senses. 2020; 45:235–48.
Article27. Nomura K, Nakanishi M, Ishidate F, Iwata K, Taruno A. All-electrical Ca2+-independent signal transduction mediates attractive sodium taste in taste buds. Neuron. 2020; 106:816–29.
Article28. Tordoff MG, Ellis HT, Aleman TR, Downing A, Marambaud P, Foskett JK, et al. Salty taste deficits in CALHM1 knockout mice. Chem Senses. 2014; 39:515–28.
Article29. Behrens M, Redel U, Blank K, Meyerhof W. The human bitter taste receptor TAS2R7 facilitates the detection of bitter salts. Biochem Biophys Res Commun. 2019; 512:877–81.
Article30. Wang Y, Zajac AL, Lei W, Christensen CM, Margolskee RF, Bouysset C, et al. Metal ions activate the human taste receptor TAS2R7. Chem Senses. 2019; 44:339–47.
Article31. Roebber JK, Roper SD, Chaudhari N. The role of the anion in salt (NaCl) detection by mouse taste buds. J Neurosci. 2019; 39:6224–32.
Article32. Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008; 586:2903–12.
Article33. Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sevigny J, Kinnamon JC, et al. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008; 33:243–54.
Article34. Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, et al. The cells and logic for mammalian sour taste detection. Nature. 2006; 442:934–8.
Article35. Zocchi D, Wennemuth G, Oka Y. The cellular mechanism for water detection in the mammalian taste system. Nat Neurosci. 2017; 20:927–33.
Article36. Wilson CE, Vandenbeuch A, Kinnamon SC. Physiological and behavioral responses to optogenetic stimulation of PKD2L1+ type III taste cells. eNeuro. 2019; 6:ENEURO.0107-19.2019.37. Zhang J, Jin H, Zhang W, Ding C, O’Keeffe S, Ye M, et al. Sour sensing from the tongue to the brain. Cell. 2019; 179:392–402.
Article38. Horio N, Yoshida R, Yasumatsu K, Yanagawa Y, Ishimaru Y, Matsunami H, et al. Sour taste responses in mice lacking PKD channels. PLoS One. 2011; 6:e20007.
Article39. Tu YH, Cooper AJ, Teng B, Chang RB, Artiga DJ, Turner HN, et al. An evolutionarily conserved gene family encodes proton-selective ion channels. Science. 2018; 359:1047–50.
Article40. Ye W, Chang RB, Bushman JD, Tu YH, Mulhall EM, Wilson CE, et al. The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc Natl Acad Sci U S A. 2016; 113:E229–38.41. Teng B, Wilson CE, Tu YH, Joshi NR, Kinnamon SC, Liman ER. Cellular and neural responses to sour stimuli require the proton channel Otop1. Curr Biol. 2019; 29:3647–56.
Article42. Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, et al. The taste of carbonation. Science. 2009; 326:443–5.
Article43. Ninomiya Y, Tonosaki K, Funakoshi M. Gustatory neural response in the mouse. Brain Res. 1982; 244:370–3.
Article44. Barretto RP, Gillis-Smith S, Chandrashekar J, Yarmolinsky DA, Schnitzer MJ, Ryba NJ, et al. The neural representation of taste quality at the periphery. Nature. 2015; 517:373–6.
Article45. Wu A, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Breadth of tuning in taste afferent neurons varies with stimulus strength. Nat Commun. 2015; 6:8171.
Article46. Lee H, Macpherson LJ, Parada CA, Zuker CS, Ryba NJP. Rewiring the taste system. Nature. 2017; 548:330–3.
Article47. Dvoryanchikov G, Hernandez D, Roebber JK, Hill DL, Roper SD, Chaudhari N. Transcriptomes and neurotransmitter profiles of classes of gustatory and somatosensory neurons in the geniculate ganglion. Nat Commun. 2017; 8:760.
Article48. Nada O, Hirata K. The occurrence of the cell type containing a specific monoamine in the taste bud of the rabbit’s foliate papila. Histochemistry. 1975; 43:237–40.
Article49. Herness MS. Vasoactive intestinal peptide-like immunoreactivity in rodent taste cells. Neuroscience. 1989; 33:411–9.
Article50. Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci U S A. 2005; 102:11100–5.
Article51. Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci. 2002; 22:10018–29.
Article52. Yu JH, Kim MS. Molecular mechanisms of appetite regulation. Diabetes Metab J. 2012; 36:391–8.
Article53. Herness S, Zhao FL. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav. 2009; 97:581–91.
Article54. Elson AE, Dotson CD, Egan JM, Munger SD. Glucagon signaling modulates sweet taste responsiveness. FASEB J. 2010; 24:3960–9.
Article55. Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008; 106:455–63.
Article56. Shin YK, Martin B, Kim W, White CM, Ji S, Sun Y, et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS One. 2010; 5:e12729.
Article57. Shigemura N, Iwata S, Yasumatsu K, Ohkuri T, Horio N, Sanematsu K, et al. Angiotensin II modulates salty and sweet taste sensitivities. J Neurosci. 2013; 33:6267–77.
Article58. Yoshida R, Noguchi K, Shigemura N, Jyotaki M, Takahashi I, Margolskee RF, et al. Leptin suppresses mouse taste cell responses to sweet compounds. Diabetes. 2015; 64:3751–62.
Article59. Calvo SS, Egan JM. The endocrinology of taste receptors. Nat Rev Endocrinol. 2015; 11:213–27.
Article60. Rohde K, Schamarek I, Bluher M. Consequences of obesity on the sense of taste: taste buds as treatment targets? Diabetes Metab J. 2020; 44:509–28.
Article61. Glendinning JI, Frim YG, Hochman A, Lubitz GS, Basile AJ, Sclafani A. Glucose elicits cephalic-phase insulin release in mice by activating KATP channels in taste cells. Am J Physiol Regul Integr Comp Physiol. 2017; 312:R597–R610.62. Doyle ME, Fiori JL, Gonzalez Mariscal I, Liu QR, Goodstein E, Yang H, et al. Insulin is transcribed and translated in mammalian taste bud cells. Endocrinology. 2018; 159:3331–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Decoding taste perception: the role of electrophysiology in taste transduction mechanisms
- Iatrogenic Taste Disorder
- Optogenetics in the study of gustatory processing: mechanisms of taste perception and neural modulation
- Immunohistochemical study on the dopaminergic and norepinephrinergic taste cells in rat taste buds
- Consequences of Obesity on the Sense of Taste: Taste Buds as Treatment Targets?