Korean J Transplant.
2021 Jun;35(2):116-123. 10.4285/kjt.20.0037.
Salvage living donor liver transplantation for post-resection recurrence of combined hepatocellular carcinoma-cholangiocarcinoma
- Affiliations
-
- 1Division of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2517295
- DOI: http://doi.org/10.4285/kjt.20.0037
Abstract
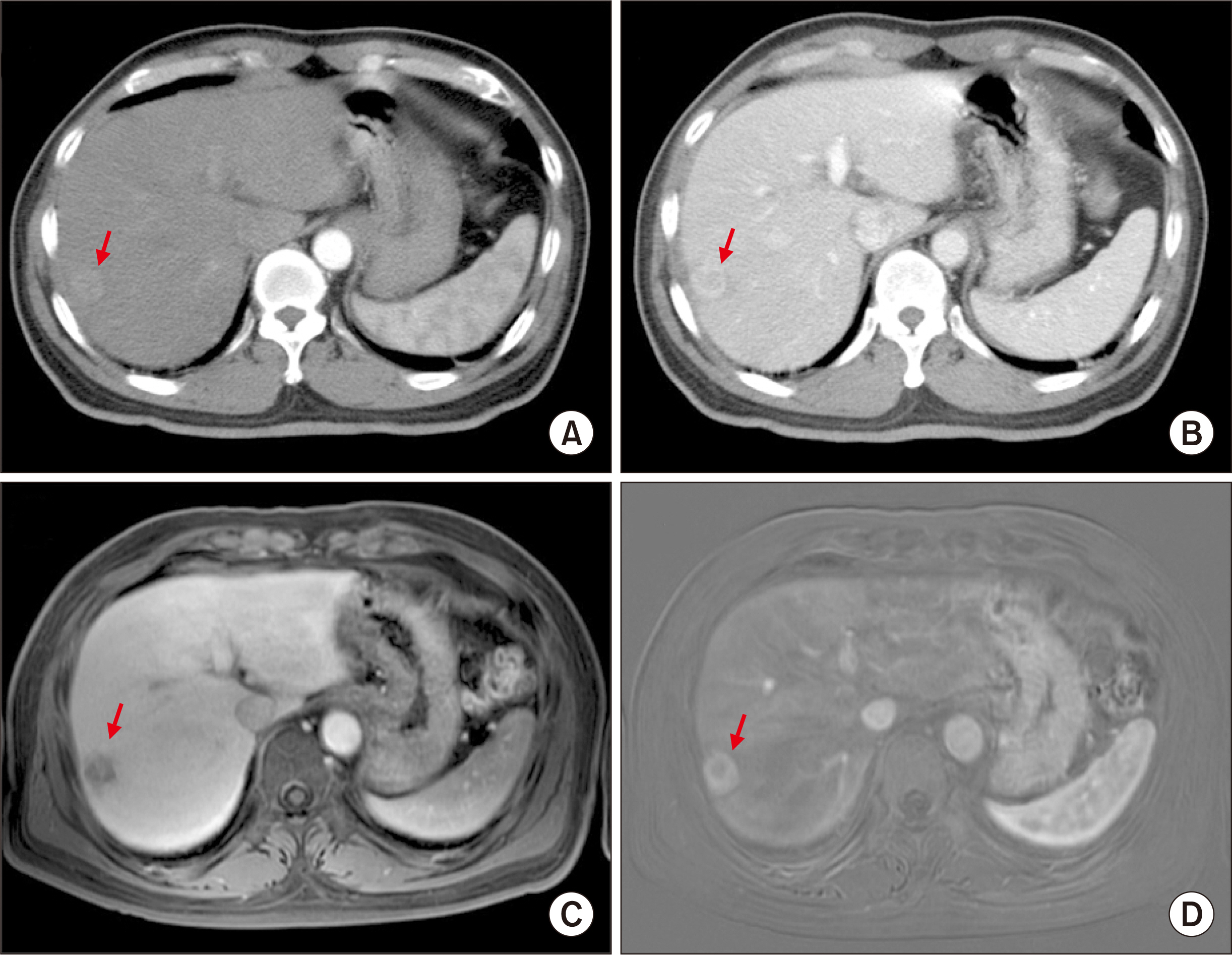
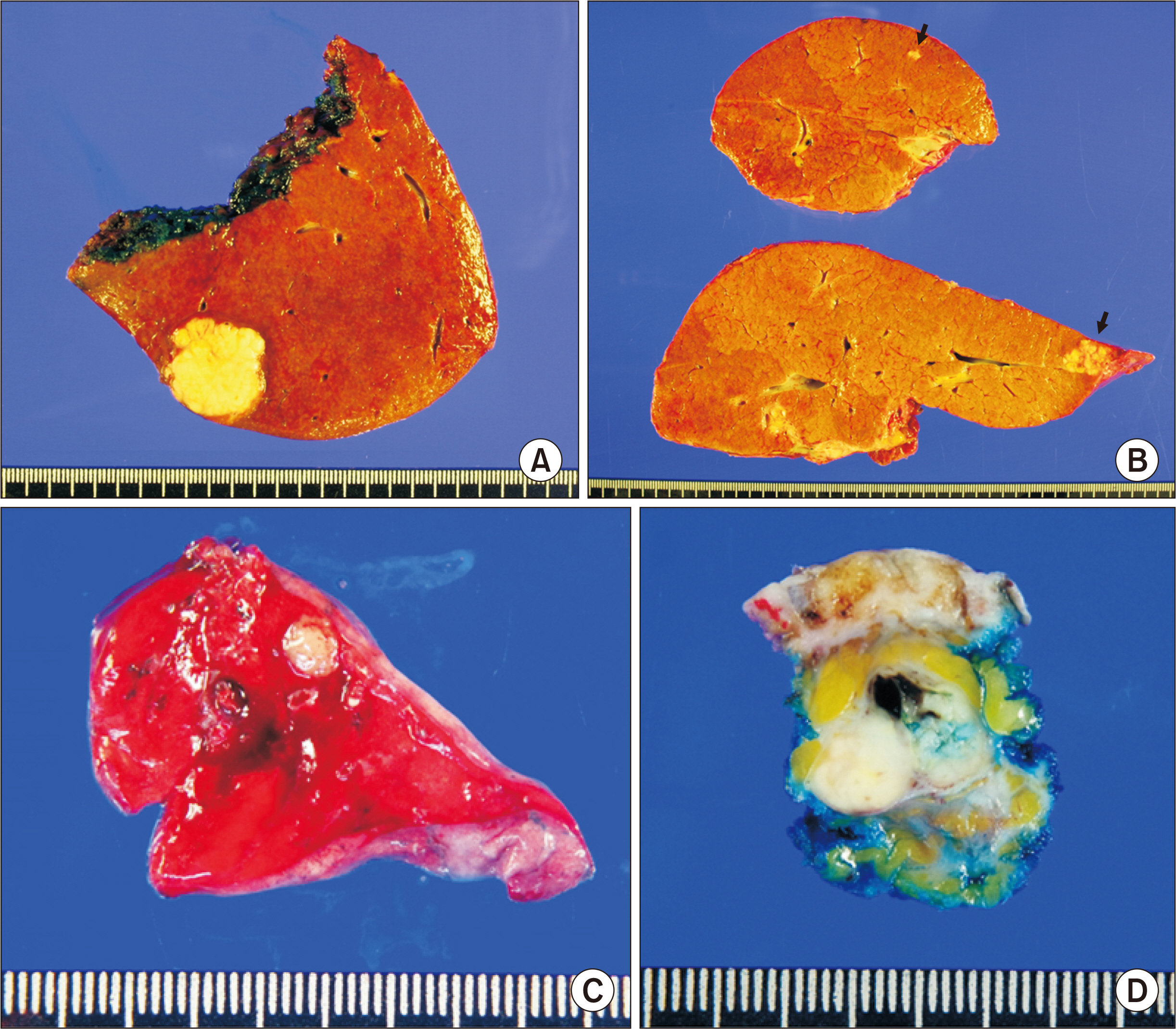
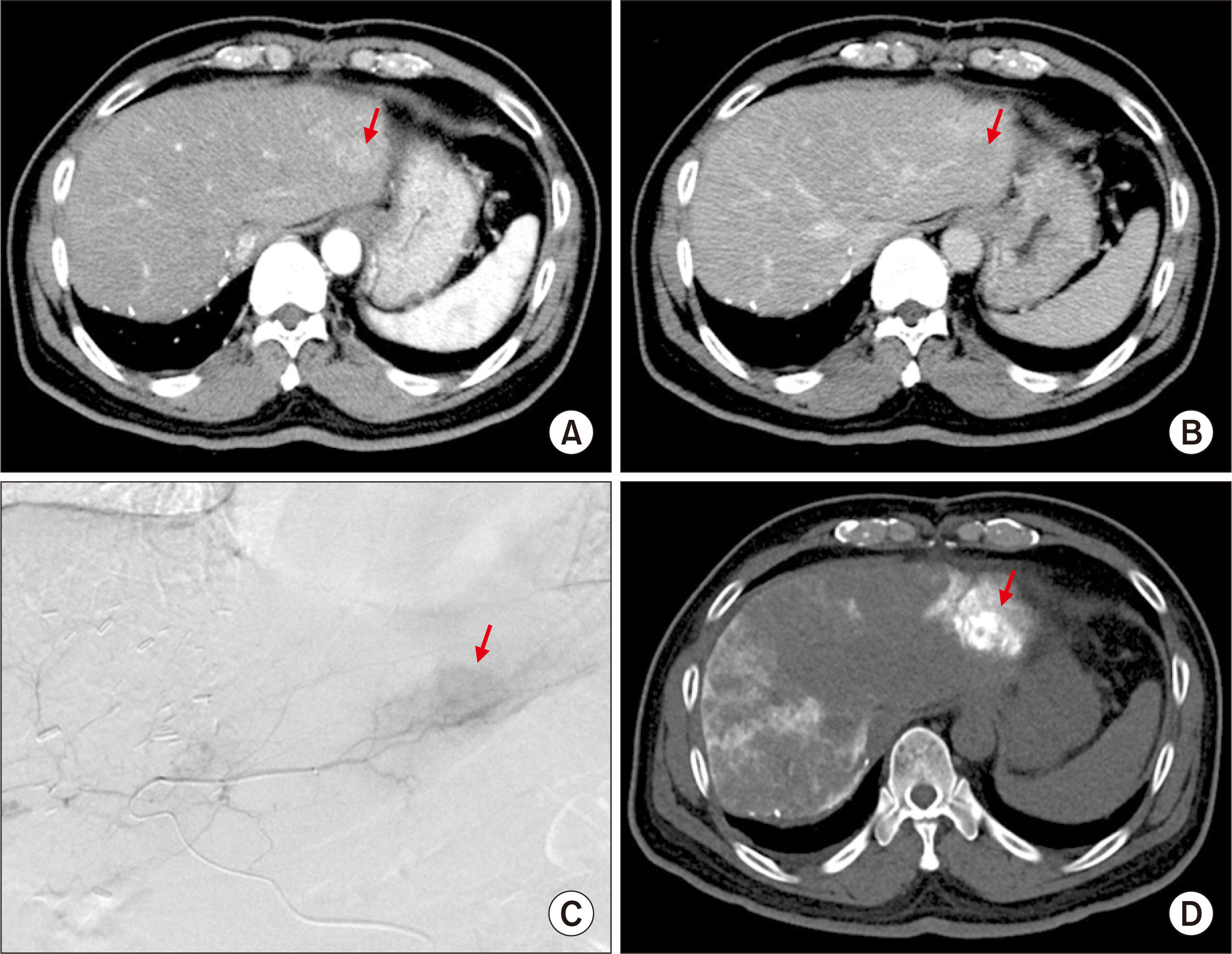
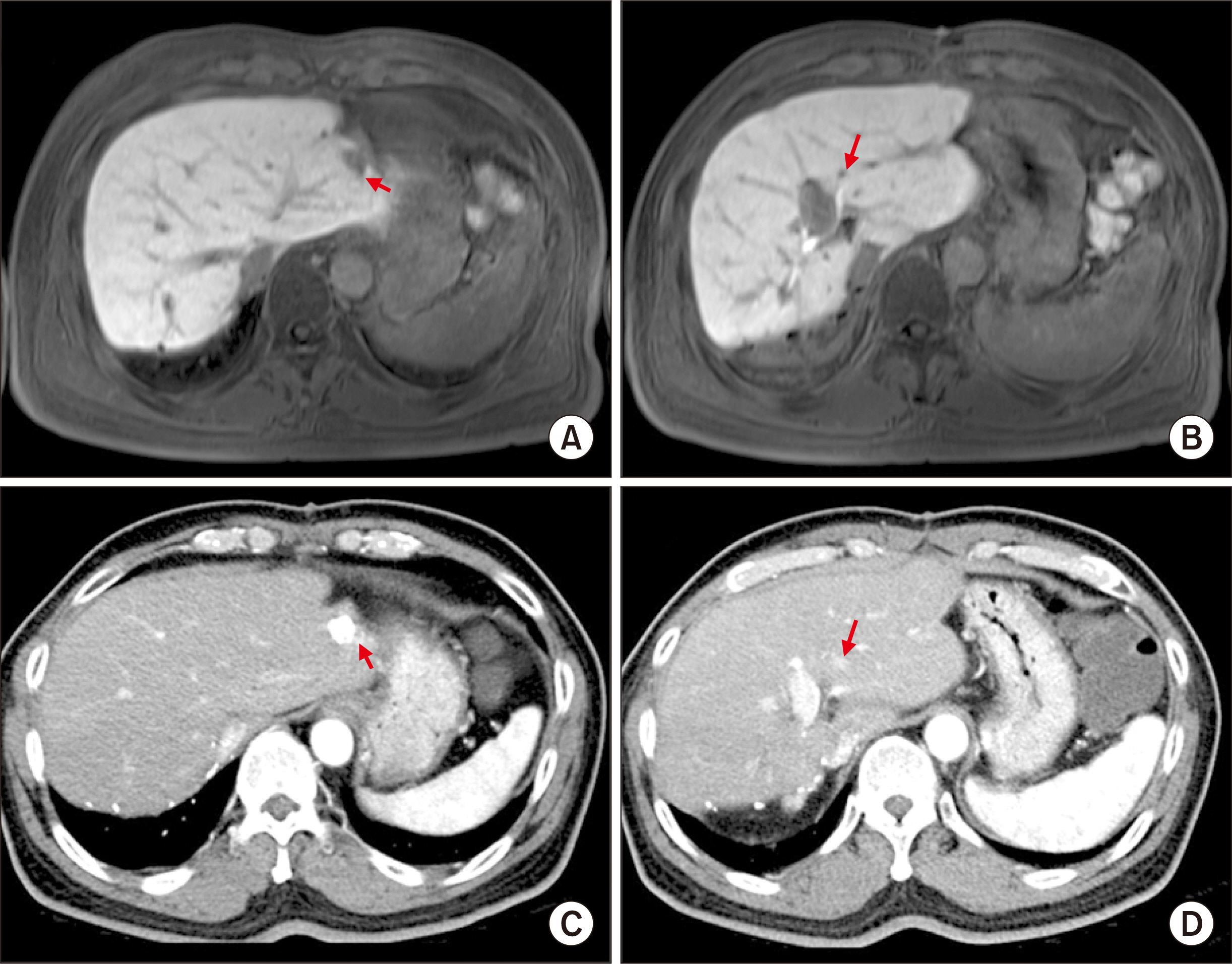
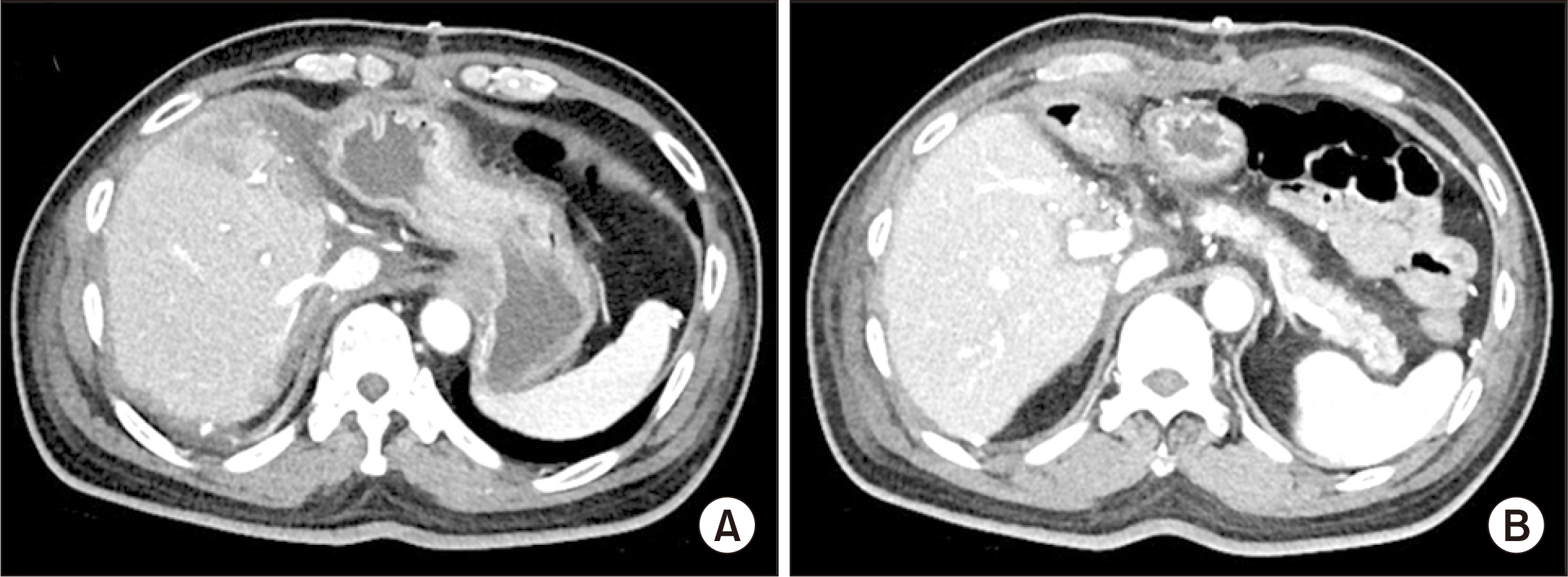
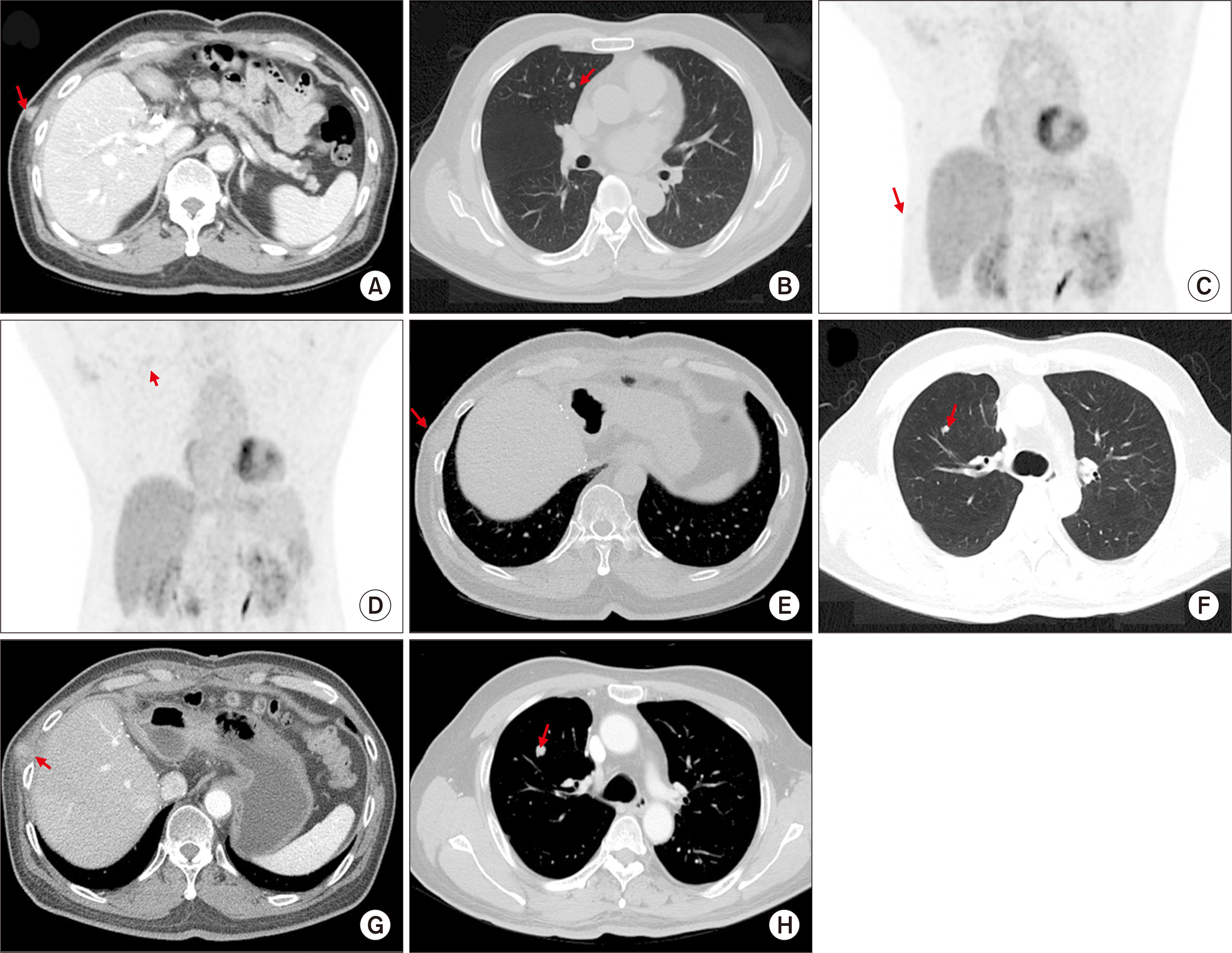
- Salvage liver transplantation (LT) is a definite treatment for recurrent hepatocellular carcinoma (HCC) after hepatectomy. Combined hepatocellular carcinoma-cholangiocarcinoma (cHCC-CC) is not eligibly indicated for LT because of high post-transplant recurrence. We present a case of salvage living donor liver transplantation (LDLT) in a patient with tumor recurrence after surgical resection of cHCC-CC. A 61-year-old male patient diagnosed with chronic hepatitis B underwent right posterior sectionectomy for HCC. The pathological diagnosis revealed presence of a 3.2-cm-sized cHCC-CC with stem cell features and intermediate cell-subtype. Intrahepatic tumor recurrence occurred 9 months later and transarterial chemoembolization was performed. Due to the progress of recurrent tumors, ABO-incompatible LDLT was performed. The explant liver pathology revealed four small masses of cHCC-CC with stem cell features. Pulmonary metastasectomy and chest wall resection were performed for metastatic lesions at 10 months after LT. Multiple tumor recurrence was detected at 21 months after LT with progression despite sorafenib administration. Currently, the patient is alive for past 26 months after LT. In conclusion, the clinical sequences of this case suggest that the role of salvage LT for cHCC-CC is much more limited than that for HCC because the tumor biology of cHCC-CC is more aggressive compared with HCC.
Figure
Reference
-
1. Jung DH, Hwang S, Song GW, Ahn CS, Moon DB, Kim KH, et al. 2017; Longterm prognosis of combined hepatocellular carcinoma-cholangiocarcinoma following liver transplantation and resection. Liver Transpl. 23:330–41. DOI: 10.1002/lt.24711. PMID: 28027599.
Article2. Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, et al. 2016; Liver transplantation for "very early" intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology. 64:1178–88. DOI: 10.1002/hep.28744. PMID: 27481548.
Article3. Jung DH, Hwang S, Hong SM, Chung YK, Song GW, Lee YJ, et al. 2017; Post-resection prognosis of combined hepatocellular carcinoma-cholangiocarcinoma according to the 2010 WHO classification. World J Surg. 41:1347–57. DOI: 10.1007/s00268-016-3837-y. PMID: 27896409.
Article4. Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, et al. 2011; Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 45:69–75. DOI: 10.1097/MCG.0b013e3181ce5dfa. PMID: 20142755.
Article5. Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, et al. 2014; Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 20:952–9. DOI: 10.1002/lt.23897. PMID: 24777610.
Article6. Theise ND, Park YN, Nakanuma Y. Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. 2010. Combined hepatocellular-cholangiocarcinoma. WHO classification of tumours of the digestive system. 4th ed. IARC;Lyon, Fr: p. 225–7.7. Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, et al. 2018; cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 68:113–26. DOI: 10.1002/hep.29789. PMID: 29360137. PMCID: PMC6340292.
Article8. Sempoux C, Kakar S, Kondo F, Schirmacher P. Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. 2019. Combined hepatocellular-cholangiocarcinoma. WHO classification of tumours: digestive system tumours. 5th ed. IARC;Lyon, Fr: p. 260–2.9. De Vito C, Sarker D, Ross P, Heaton N, Quaglia A. 2017; Histological heterogeneity in primary and metastatic classic combined hepatocellular-cholangiocarcinoma: a case series. Virchows Arch. 471:619–29. DOI: 10.1007/s00428-017-2196-x. PMID: 28707055.
Article10. Kim JH, Yoon HK, Ko GY, Gwon DI, Jang CS, Song HY, et al. 2010; Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology. 255:270–7. DOI: 10.1148/radiol.09091076. PMID: 20308463.
Article11. Na SK, Choi GH, Lee HC, Shin YM, An J, Lee D, et al. 2018; The effectiveness of transarterial chemoembolization in recurrent hepatocellular-cholangiocarcinoma after resection. PLoS One. 13:e0198138. DOI: 10.1371/journal.pone.0198138. PMID: 29879137. PMCID: PMC5991684.
Article12. Kobayashi S, Terashima T, Shiba S, Yoshida Y, Yamada I, Iwadou S, et al. 2018; Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 109:2549–57. DOI: 10.1111/cas.13656. PMID: 29856900. PMCID: PMC6113439.
Article13. Kang SH, Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, et al. 2019; Cross-sectional analysis of immunosuppressive regimens focused on everolimus after liver transplantation in a Korean high-volume transplantation center. Korean J Transplant. 33:98–105. DOI: 10.4285/jkstn.2019.33.4.98.
Article14. Yamanaka K, Petrulionis M, Lin S, Gao C, Galli U, Richter S, et al. 2013; Therapeutic potential and adverse events of everolimus for treatment of hepatocellular carcinoma: systematic review and meta-analysis. Cancer Med. 2:862–71. DOI: 10.1002/cam4.150. PMID: 24403259. PMCID: PMC3892390.15. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. 2017. AJCC cancer staging manual. 8th ed. Springer;New York, NY: DOI: 10.1007/978-3-319-40618-3_2.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Usefulness of Salvage Transplantation in Patients with Hepatocellular Carcinoma
- Resection or transplantation for perihilar cholangiocarcinoma
- Assessment of Technical Feasibility of Living Donor Liver Transplantation after Prior Major Liver Resection for Hepatocellular Carcinoma
- Venous outflow congestion is related to poor recurrence-free survival of living donor liver transplantation recipients with hepatocellular carcinoma
- Liver Transplantation for Hepatocellular Carcinoma