Neonatal Med.
2021 May;28(2):65-71. 10.5385/nm.2021.28.2.65.
Influence of Postconceptional Age on the Renal Biomarkers in Very-Low-Birth-Weight Infants
- Affiliations
-
- 1Department of Pediatrics, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea
- 2Department of Urology, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea
- KMID: 2516487
- DOI: http://doi.org/10.5385/nm.2021.28.2.65
Abstract
- Purpose
We investigated whether consecutive levels of new emerging renal biomarkers, including serum cystatin C (CysC) and urinary neutrophil gelatinase-associated lipocalin (NGAL)/creatinine (Cr) ratio, were affected by postconceptional age in very-low-birth-weight (VLBW) infants.
Methods
Repeatedly measured samples for each infant were divided into four groups according to postnatal age: at birth (stage I), 3 to 7 days postnatally (stage II), 8 to 28 days postnatally (stage III), and >28 days postnatally (stage IV). The association between renal biomarkers and postconceptional age was assessed using Pearson’s correlation coefficient, and the mean values of renal biomarkers in the four stages were compared using repeated-measures analysis of variance.
Results
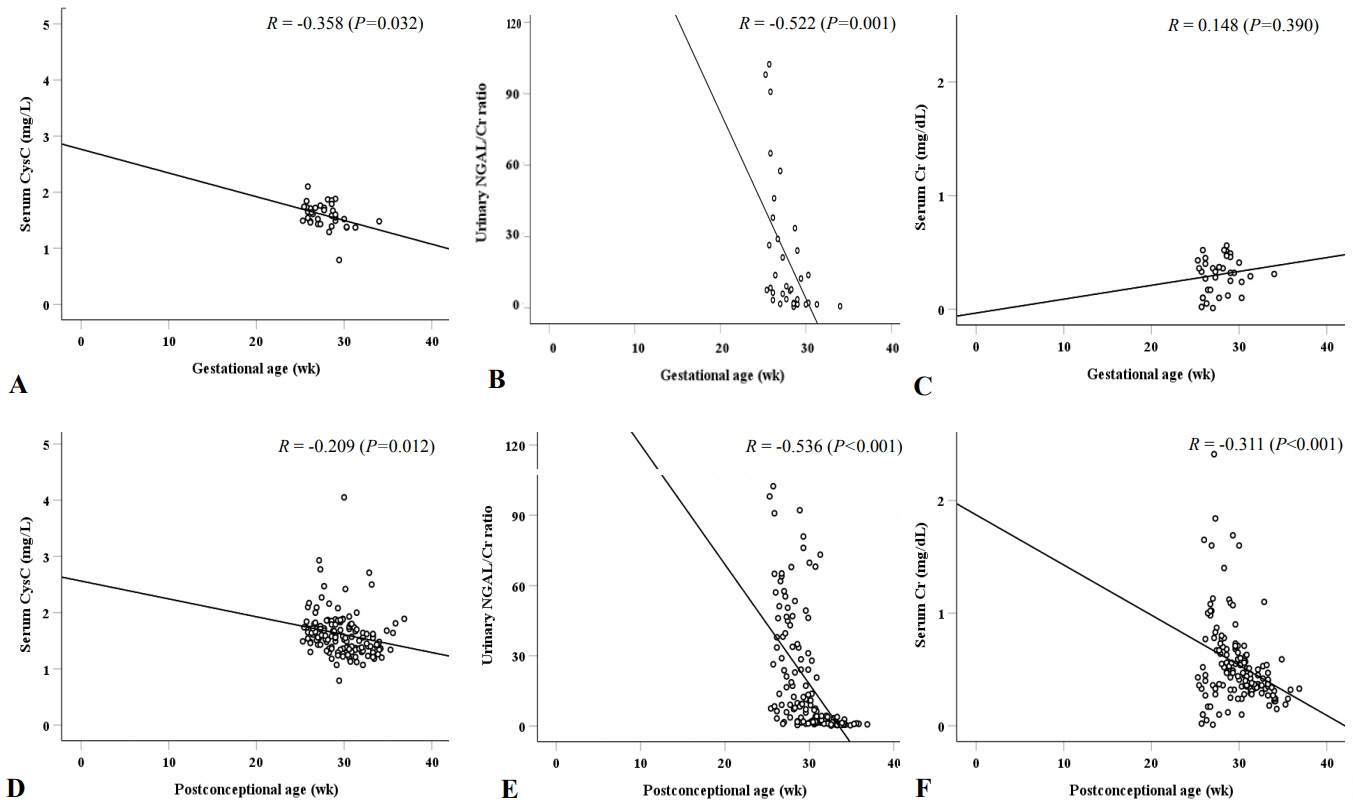
For samples measured at birth, serum CysC (r=–0.358, P=0.032) and urinary NGAL/Cr ratio (r=–0.522, P=0.001) were negatively correlated with gestational age, whereas serum Cr (r=0.148, P=0.390) was not. In addition, for all samples measured, serum CysC (r=–0.209, P=0.012), urinary NGAL/Cr ratio (r=–0.536, P<0.001), and serum Cr (r=–0.311, P<0.001) were negatively correlated with postconceptional age. Compared with the mean values of the postnatal age-specific stages, serum CysC showed no significant differences in any of the four stages. However, the urinary NGAL/Cr ratio in stage IV was significantly different from those in stages I to III.
Conclusion
Although urinary NGAL/Cr ratio and serum CysC were negatively correlated with postconceptional age considering renal development, serum CysC showed no significant differences in any of the four postnatal age-specific stages. Urinary NGAL/Cr ratio at >28 days postnatally seems to be more affected by postconceptional age than serum CysC in VLBW infants.
Figure
Reference
-
1. Askenazi DJ, Griffin R, McGwin G, Carlo W, Ambalavanan N. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol. 2009; 24:991–7.2. Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. 2011; 69:354–8.3. Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991; 64:777–84.4. Stelloh C, Allen KP, Mattson DL, Lerch-Gaggl A, Reddy S, El-Meanawy A. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl Res. 2012; 159:80–9.5. Veille JC, McNeil S, Hanson R, Smith N. Renal hemodynamics: longitudinal study from the late fetal life to one year of age. J Matern Fetal Investig. 1998; 8:6–10.6. Rhone ET, Carmody JB, Swanson JR, Charlton JR. Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med. 2014; 27:1485–90.7. Barhight M, Altaye M, Gist KM, Isemann B, Goldstein SL, Akinbi H. Nephrotoxic medications and associated acute kidney injury in very low birth weight infants. J Clin Nephrol Res. 2017; 4:1070.8. Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, et al. Neonatal acute kidney injury. Pediatrics. 2015; 136:e463–73.9. Filler G, Browne R, Seikaly MG. Glomerular filtration rate as a putative ‘surrogate end-point’ for renal transplant clinical trials in children. Pediatr Transplant. 2003; 7:18–24.10. Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know?: what do we need to learn? Pediatr Nephrol. 2009; 24:265–74.11. Gordjani N, Burghard R, Leititis JU, Brandis M. Serum creatinine and creatinine clearance in healthy neonates and prematures during the first 10 days of life. Eur J Pediatr. 1988; 148:143–5.12. Iacobelli S, Bonsante F, Ferdinus C, Labenne M, Gouyon JB. Factors affecting postnatal changes in serum creatinine in preterm infants with gestational age <32 weeks. J Perinatol. 2009; 29:232–6.13. Filler GM. The challenges of assessing acute kidney injury in infants. Kidney Int. 2011; 80:567–8.14. Gallini F, Maggio L, Romagnoli C, Marrocco G, Tortorolo G. Progression of renal function in preterm neonates with gestational age < or = 32 weeks. Pediatr Nephrol. 2000; 15:119–24.15. Delanaye P, Ebert N. Assessment of kidney function: Estimating GFR in children. Nat Rev Nephrol. 2012; 8:503–4.16. Demirel G, Celik IH, Canpolat FE, Erdeve O, Biyikli Z, Dilmen U. Reference values of serum cystatin C in very low-birthweight premature infants. Acta Paediatr. 2013; 102:e4–7.17. Elmas AT, Tabel Y, Elmas ON. Reference intervals of serum cystatin C for determining cystatin C-based glomerular filtration rates in preterm neonates. J Matern Fetal Neonatal Med. 2013; 26:1474–8.18. Kristensen K, Strevens H, Lindstrom V, Grubb A, Wide-Swensson D. Increased plasma levels of beta2-microglobulin, cystatin C and beta-trace protein in term pregnancy are not due to uteroplacental production. Scand J Clin Lab Invest. 2008; 68:649–53.19. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002; 40:221–6.20. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003; 14:2534–43.21. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005; 115:610–21.22. Lavery AP, Meinzen-Derr JK, Anderson E, Ma Q, Bennett MR, Devarajan P, et al. Urinary NGAL in premature infants. Pediatr Res. 2008; 64:423–8.23. Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007; 11:R84.24. Devarajan P. NGAL for the detection of acute kidney injury in the emergency room. Biomark Med. 2014; 8:217–9.25. Shin SY, Ha JY, Lee SL, Lee WM, Park JH. Increased urinary neutrophil gelatinase-associated lipocalin in very-low-birth-weight infants with oliguria and normal serum creatinine. Pediatr Nephrol. 2017; 32:1059–65.26. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978; 187:1–7.27. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–34.28. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163:1723–9.29. Osberg IM, Hammond KB. A solution to the problem of bilirubin interference with the kinetic Jaffé method for serum creatinine. Clin Chem. 1978; 24:1196–7.30. Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010; 78:486–94.31. Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol. 2011; 22:1365–74.32. Bueva A, Guignard JP. Renal function in preterm neonates. Pediatr Res. 1994; 36:572–7.33. Kuppens M, George I, Lewi L, Levtchenko E, Allegaert K. Creatinaemia at birth is equal to maternal creatinaemia at delivery: does this paradigm still hold? J Matern Fetal Neonatal Med. 2012; 25:978–80.34. Cataldi L, Mussap M, Bertelli L, Ruzzante N, Fanos V, Plebani M. Cystatin C in healthy women at term pregnancy and in their infant newborns: relationship between maternal and neonatal serum levels and reference values. Am J Perinatol. 1999; 16:287–95.35. Kandasamy Y, Smith R, Wright IM. Measuring cystatin C to determine renal function in neonates. Pediatr Crit Care Med. 2013; 14:318–22.36. Huynh TK, Bateman DA, Parravicini E, Lorenz JM, Nemerofsky SL, Sise ME, et al. Reference values of urinary neutrophil gelatinase- associated lipocalin in very low birth weight infants. Pediatr Res. 2009; 66:528–32.37. Gubhaju L, Sutherland MR, Horne RS, Medhurst A, Kent AL, Ramsden A, et al. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Renal Physiol. 2014; 307:F149–58.38. Lee JH, Hahn WH, Ahn J, Chang JY, Bae CW. Serum cystatin C during 30 postnatal days is dependent on the postconceptional age in neonates. Pediatr Nephrol. 2013; 28:1073–8.39. Parravicini E, Lorenz JM, Nemerofsky SL, O’Rourke M, Barasch J, Bateman D. Reference range of urinary neutrophil gelatinase-associated lipocalin in very low-birth-weight infants: preliminary data. Am J Perinatol. 2009; 26:437–40.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Screening Examination for Retinopathy of Prematurity with Dual Parameter Protocol
- The Optimal Time of First Ophthalmic Examination for Retinopathy of Prematurity Screening in Extremely Low Birth Weight Infants
- Study of the Normal Value of Neonatal Blood Pressure according to Postconceptional Age
- Clinical Study of Prematurity and Low Birth Weight Infants
- Incidence and Risk Factors of Retinopathy of Prematurity in Extremely Low Birth Weight and Very Low Birth Weight Infants