Acute Crit Care.
2021 May;36(2):85-91. 10.4266/acc.2021.00150.
Up-to-date information on polymyxin B-immobilized fiber column direct hemoperfusion for septic shock
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Juntendo University Faculty of Medicine, Tokyo, Japan
- 2Department of Internal Medicine and Rheumatology, Juntendo University Faculty of Medicine, Tokyo, Japan
- KMID: 2516255
- DOI: http://doi.org/10.4266/acc.2021.00150
Abstract
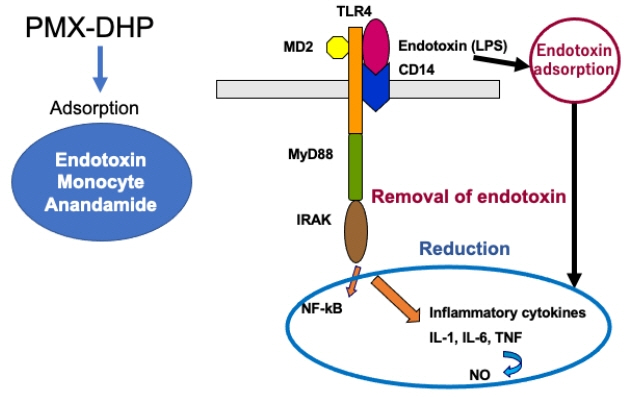
- Endotoxin adsorption therapy by polymyxin B-immobilized fiber column direct hemoperfusion (PMX-DHP) has been used for the treatment of septic shock patients. Endotoxin, an outer membrane component of Gram-negative bacteria, plays an important role in the pathogenesis of septic shock. Endotoxin triggers a signaling cascade for leukocytes, macrophage, and endothelial cells to secrete various mediators including cytokines and nitric oxide, leading to septic shock and multiple organ dysfunction syndrome. PMX-DHP directly adsorbed not only endotoxin but also monocytes and anandamide. It reduced blood levels of inflammatory cytokines such as interleukin (IL)-1, IL-6, tumor necrosis factor-alpha and IL-17A, adhesion molecules, plasminogen activator inhibitor 1, and high mobility group box-1. As a result, PMX-DHP increased blood pressure and reduced the dose of vasoactive-inotropic agents. PMX-DHP improved monocyte human leukocyte antigen-DR expression in patients with severe sepsis and septic shock. A post hoc analysis of EUPHRATES (Evaluating the Use of Polymyxin B Hemoperfusion in Randomized Controlled Trial of Adults Treated for Endotoxemia and Septic Shock) trial has shown that PMX-DHP significantly reduced 28-day mortality compared with the control group in septic shock patients with endotoxin activity assay level between 0.60 and 0.89. Longer duration of PMX-DHP may be another strategy to bring out the beneficial effects of PMX-DHP. Further studies are needed to confirm the efficacy of PMX-DHP treatment for septic shock.
Keyword
Figure
Reference
-
1. Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000; 406:782–7.
Article2. Reinhart K, Bayer O, Brunkhorst F, Meisner M. Markers of endothelial damage in organ dysfunction and sepsis. Crit Care Med. 2002; 30(5 Suppl):S302–12.
Article3. Tsujimoto H, Ono S, Hiraki S, Majima T, Kawarabayashi N, Sugasawa H, et al. Hemoperfusion with polymyxin B-immobilized fibers reduced the number of CD16+ CD14+ monocytes in patients with septic shock. J Endotoxin Res. 2004; 10:229–37.4. Nishibori M, Takahashi HK, Katayama H, Mori S, Saito S, Iwagaki H, et al. Specific removal of monocytes from peripheral blood of septic patients by polymyxin B-immobilized filter column. Acta Med Okayama. 2009; 63:65–9.5. Wang Y, Liu Y, Sarker KP, Nakashima M, Serizawa T, Kishida A, et al. Polymyxin B binds to anandamide and inhibits its cytotoxic effect. FEBS Lett. 2000; 470:151–5.
Article6. Nakamura T, Ebihara I, Shoji H, Ushiyama C, Suzuki S, Koide H. Treatment with polymyxin B-immobilized fiber reduces platelet activation in septic shock patients: decrease in plasma levels of soluble P-selectin, platelet factor 4 and beta-thromboglobulin. Inflamm Res. 1999; 48:171–5.7. Nemoto H, Nakamoto H, Okada H, Sugahara S, Moriwaki K, Arai M, et al. Newly developed immobilized polymyxin B fibers improve the survival of patients with sepsis. Blood Purif. 2001; 19:361–8.
Article8. Nakamura T, Matsuda T, Suzuki Y, Shoji H, Koide H. Polymyxin B-immobilized fiber hemoperfusion in patients with sepsis. Dial Tranplant. 2003; 32:602–7.9. Vincent JL, Laterre PF, Cohen J, Burchardi H, Bruining H, Lerma FA, et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock. 2005; 23:400–5.
Article10. Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009; 301:2445–52.11. Suzuki H, Nemoto H, Nakamoto H, Okada H, Sugahara S, Kanno Y, et al. Continuous hemodiafiltration with polymyxin-B immobilized fiber is effective in patients with sepsis syndrome and acute renal failure. Ther Apher. 2002; 6:234–40.
Article12. Tani T, Hanasawa K, Kodama M, Imaizumi H, Yonekawa M, Saito M, et al. Correlation between plasma endotoxin, plasma cytokines, and plasminogen activator inhibitor-1 activities in septic patients. World J Surg. 2001; 25:660–8.
Article13. Ikeda T, Ikeda K, Nagura M, Taniuchi H, Matsushita M, Kiuchi S, et al. Clinical evaluation of PMX-DHP for hypercytokinemia caused by septic multiple organ failure. Ther Apher Dial. 2004; 8:293–8.
Article14. Mitaka C, Tsuchida N, Kawada K, Nakajima Y, Imai T, Sasaki S. A longer duration of polymyxin B-immobilized fiber column hemoperfusion improves pulmonary oxygenation in patients with septic shock. Shock. 2009; 32:478–83.
Article15. Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Maeda S, Yamagishi S. Suppression of high-mobility group box-1 and receptor for advanced glycation end-product axis by polymyxin B-immobilized fiber hemoperfusion in septic shock patients. J Crit Care. 2011; 26:546–9.
Article16. Sugiura M, Mitaka C, Haraguchi G, Tomita M, Inase N. Polymyxin B-immobilized fiber column hemoperfusion mainly helps to constrict peripheral blood vessels in treatment for septic shock. J Intensive Care. 2015; 3:14.
Article17. Nakamura T, Kawagoe Y, Matsuda T, Koide H. Effect of polymyxin B-immobilized fiber on bone resorption in patients with sepsis. Intensive Care Med. 2004; 30:1838–41.
Article18. Kushi H, Miki T, Okamaoto K, Nakahara J, Saito T, Tanjoh K. Early hemoperfusion with an immobilized polymyxin B fiber column eliminates humoral mediators and improves pulmonary oxygenation. Crit Care. 2005; 9:R653–61.19. Ueno T, Ikeda T, Ikeda K, Taniuchi H, Suda S, Yeung MY, et al. HMGB-1 as a useful prognostic biomarker in sepsis-induced organ failure in patients undergoing PMX-DHP. J Surg Res. 2011; 171:183–90.
Article20. Kushi H, Miki T, Nakahara J, Okamoto K, Saito T, Tanjoh K. Hemoperfusion with an immobilized polymyxin B column reduces the blood level of neutrophil elastase. Blood Purif. 2006; 24:212–7.
Article21. Coudroy R, Payen D, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, et al. Modulation by polymyxin-B hemoperfusion of inflammatory response related to severe peritonitis. Shock. 2017; 47:93–9.
Article22. Bosmann A, Ward PA. Therapeutic potential of targeting IL17 and IL-23 in sepsis. Clin Transl Med. 2012; 1:4.
Article23. Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008; 22:2198–205.
Article24. Cantaluppi V, Assenzio B, Pasero D, Romanazzi GM, Pacitti A, Lanfranco G, et al. Polymyxin-B hemoperfusion inactivates circulating proapoptotic factors. Intensive Care Med. 2008; 34:1638–45.
Article25. Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007; 33:409–13.
Article26. Ono S, Tsujimoto H, Matsumoto A, Ikuta S, Kinoshita M, Mochizuki H. Modulation of human leukocyte antigen-DR on monocytes and CD16 on granulocytes in patients with septic shock using hemoperfusion with polymyxin B-immobilized fiber. Am J Surg. 2004; 188:150–6.
Article27. Monneret G, Lepape A, Voirin N, Bohé J, Venet F, Debard AL, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006; 32:1175–83.
Article28. Srisawat N, Tungsanga S, Lumlertgul N, Komaenthammasophon C, Peerapornratana S, Thamrongsat N, et al. The effect of polymyxin B hemoperfusion on modulation of human leukocyte antigen DR in severe sepsis patients. Crit Care. 2018; 22:279.
Article29. Wittkowski H, Sturrock A, van Zoelen MA, Viemann D, van der Poll T, Hoidal JR, et al. Neutrophil-derived S100A12 in acute lung injury and respiratory distress syndrome. Crit Care Med. 2007; 35:1369–75.
Article30. Takahashi G, Hoshikawa K, Matsumoto N, Shozushima T, Onodera C, Kan S, et al. Changes in serum S100A12 and sRAGE associated with improvement of the PaO2/FiO2 ratio following PMX-DHP therapy for postoperative septic shock. Eur Surg Res. 2011; 47:135–40.31. Payen DM, Guilhot J, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015; 41:975–84.
Article32. Dellinger RP, Bagshaw SM, Antonelli M, Foster DM, Klein DJ, Marshall JC, et al. Septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA. 2018; 320:1455–63.33. Klein DJ, Foster D, Walker PM, Bagshaw SM, Mekonnen H, Antonelli M. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 2018; 44:2205–12.
Article34. Shoji H. Extracorporeal endotoxin removal for the treatment of sepsis: endotoxin adsorption cartridge (Toraymyxin). Ther Apher Dial. 2003; 7:108–14.35. Yamashita C, Hara Y, Kuriyama N, Nakamura T, Nishida O. Clinical effects of a longer duration of polymyxin B-immobilized fiber column direct hemoperfusion therapy for severe sepsis and septic shock. Ther Apher Dial. 2015; 19:316–23.
Article36. Miyamoto K, Kawazoe Y, Kato S. Prolonged direct hemoperfusion using a polymyxin B immobilized fiber cartridge provides sustained circulatory stabilization in patients with septic shock: a retrospective observational before-after study. J Intensive Care. 2017; 5:19.
Article37. Kawazoe Y, Sato T, Miyagawa N, Yokokawa Y, Kushimoto S, Miyamoto K, et al. Mortality effects of prolonged hemoperfusion therapy using a polymyxin B-immobilized fiber column for patients with septic shock: a sub-analysis of the DESIRE trial. Blood Purif. 2018; 46:309–14.
Article38. Mitaka C, Fujiwara N, Yamamoto M, Toyofuku T, Haraguchi G, Tomita M. Polymyxin B-immobilized fiber column hemoperfusion removes endotoxin throughout a 24-hour treatment period. J Crit Care. 2014; 29:728–32.
Article39. Berto P, Ronco C, Cruz D, Melotti RM, Antonelli M. Cost-effectiveness analysis of polymyxin-B immobilized fiber column and conventional medical therapy in the management of abdominal septic shock in Italy. Blood Purif. 2011; 32:331–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A successful application of adult polymyxin B-immobilized fiber column hemoperfusion to a neonate with septic shock
- Polymyxin B Immobilized Fiber Hemoperfusion in Refractory Intra-abdominal Septic Shock
- Refractory Septic Shock Treated with Nephrectomy under the Support of Extracorporeal Membrane Oxygenation
- Polymyxin B Hemoperfusion in Pneumonic Septic Shock Caused by Gram-Negative Bacteria
- Suggestions and tips regarding polymyxin B-immobilized fiber column direct hemoperfusion of neonates with sepsis