Nutr Res Pract.
2021 Jun;15(3):294-308. 10.4162/nrp.2021.15.3.294.
Allomyrina dichotoma larva extract attenuates free fatty acid-induced lipotoxicity in pancreatic beta cells
- Affiliations
-
- 1Department of Food Nutrition, College of Bio Convergence, Eulji University, Seongnam 13135, Korea
- 2Institute of Lee Gil Ya Cancer and Diabetes, Department of Molecular Medicine, Gachon University, Incheon 21999, Korea
- 3College of Pharmacy and Natural Medicine Research Institute, Mokpo National University, Muan 58554, Korea
- 4College of Pharmacy, Hanyang University, Ansan 15588, Korea
- 5College of Pharmacy and Gachon Institute of Pharmaceutical Science, Gachon University, Incheon 21936, Korea
- KMID: 2516069
- DOI: http://doi.org/10.4162/nrp.2021.15.3.294
Abstract
- BACKGROUD/OBJECTIVES: Allomyrina dichotoma larva (ADL), one of the many edible insects recognized as future food resources, has a range of pharmacological activities. In a previous study, an ADL extract (ADLE) reduced the hepatic insulin resistance of high-fat diet (HFD)-induced diabetic mice. On the other hand, the associated molecular mechanisms underlying pancreatic beta-cell dysfunction remain unclear. This study examined the effects of ADLE on palmitate-induced lipotoxicity in a beta cell line of a rat origin, INS-1 cells.
MATERIALS/METHODS
ADLE was administered to high-fat diet treated mice. The expression of apoptosis-related molecules was measured by Western blotting, and reactive oxidative stress generation and nitric oxide production were measured by DCH-DA fluorescence and a Griess assay, respectively.
RESULTS
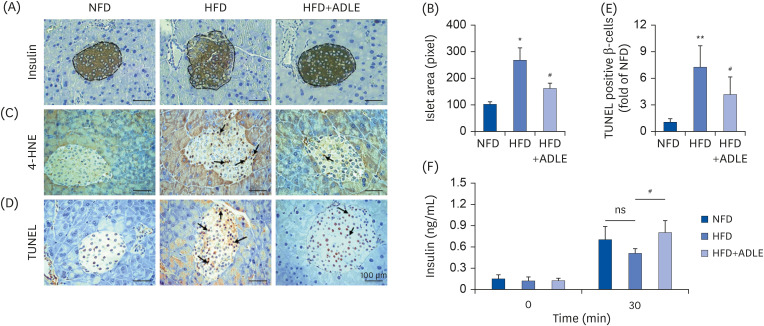
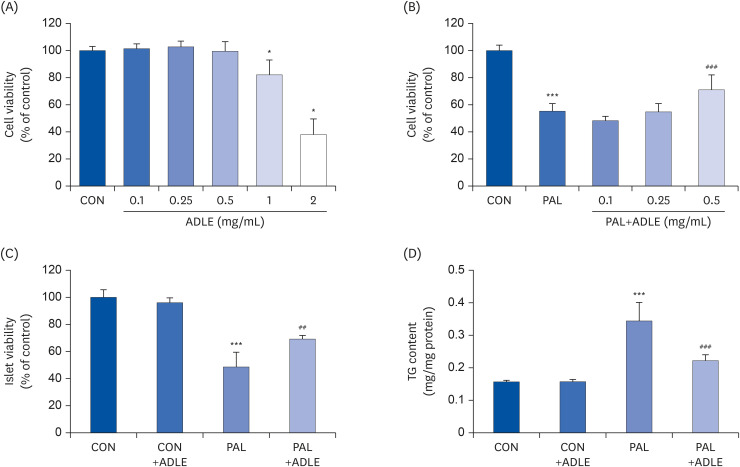
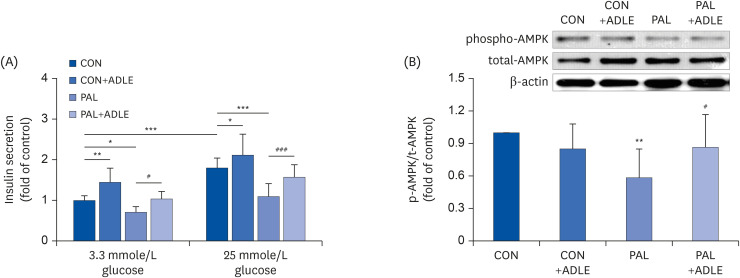
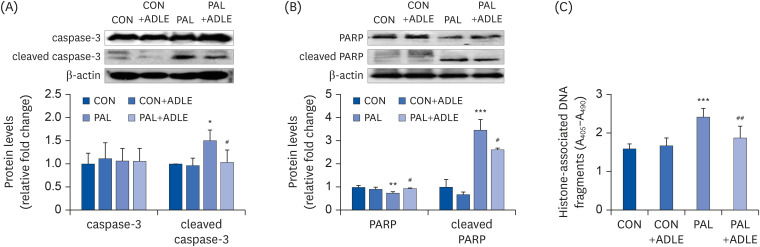
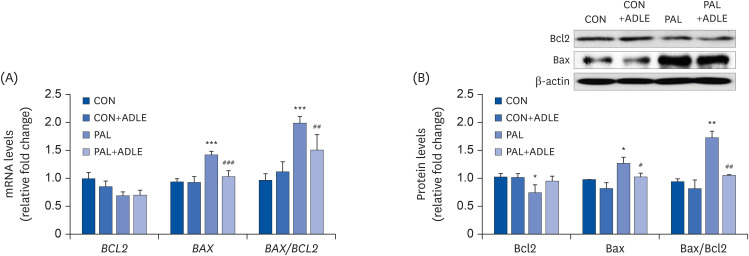
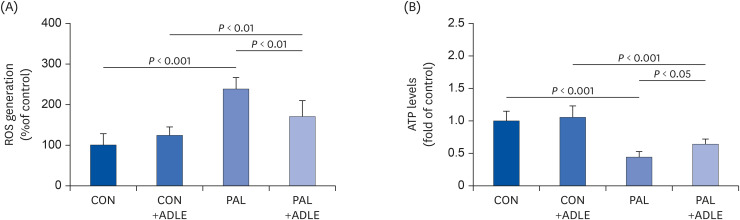
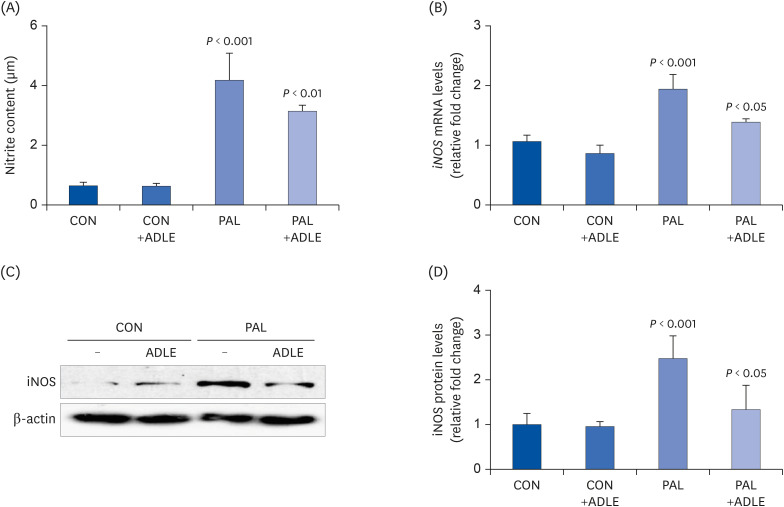
The administration of ADLE to HFD-induced diabetic mice reduced the hyperplasia, 4-hydroxynonenal levels, and the number of apoptotic cells while improving the insulin levels compared to the HFD group. Treatment of INS-1 cells with palmitate reduced insulin secretion, which was attenuated by the ADLE treatment. Furthermore, the ADLE treatment prevented palmitate-induced cell death in INS-1 cells and isolated islets by reducing the apoptotic signaling molecules, including cleaved caspase-3 and PARP, and the Bax/Bcl2 ratio. ADLE also reduced the levels of reactive oxygen species generation, lipid accumulation, and nitrite production in palmitate-treated INS-1 cells while increasing the ATP levels. This effect corresponded to the decreased expression of inducible nitric oxide synthase (iNOS) mRNA and protein.
CONCLUSIONS
ADLE helps prevent lipotoxic beta-cell death in INS-1 cells and HFD-diabetic mice, suggesting that ADLE can be used to prevent or treat beta-cell damage in glucose intolerance during the development of diabetes.
Figure
Reference
-
1. Leonardi O, Mints G, Hussain MA. Beta-cell apoptosis in the pathogenesis of human type 2 diabetes mellitus. Eur J Endocrinol. 2003; 149:99–102. PMID: 12887285.
Article2. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004; 116:205–219. PMID: 14744432.3. Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005; 5:189–200. PMID: 15719025.
Article4. Wong WW, Puthalakath H. Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway. IUBMB Life. 2008; 60:390–397. PMID: 18425793.
Article5. Oh YS, Bae GD, Baek DJ, Park EY, Jun HS. Fatty acid-induced lipotoxicity in pancreatic beta-bells during development of type 2 diabetes. Front Endocrinol (Lausanne). 2018; 9:384. PMID: 30061862.
Article6. Pemberton RW. Insects and other arthropods used as drugs in Korean traditional medicine. J Ethnopharmacol. 1999; 65:207–216. PMID: 10404418.
Article7. Meyer-Rochow VB. Ethno-entomological observations from North Korea (officially known as the “Democratic People's Republic of Korea”). J Ethnobiol Ethnomed. 2013; 9:7. PMID: 23324196.
Article8. Kim DS, Huh J, You GC, Chae SC, Lee OS, Lee HB, Lee JB, Kim JS. Allomyrina Dichotoma Larva extracts protect streptozotocin-induced oxidative cytotoxicity. J Environ Toxicol. 2007; 22:349–355.9. Sagisaka A, Miyanoshita A, Ishibashi J, Yamakawa M. Purification, characterization and gene expression of a glycine and proline-rich antibacterial protein family from larvae of a beetle, Allomyrina dichotoma . Insect Mol Biol. 2001; 10:293–302. PMID: 11520352.10. Kim K, Bae GD, Lee M, Park EY, Baek DJ, Kim CY, Jun HS, Oh YS. Allomyrina dichotoma Larva extract ameliorates the hepatic insulin resistance of high-fat diet-induced diabetic mice. Nutrients. 2019; 11:1522.11. Bégin-Heick N. Of mice and women: the beta 3-adrenergic receptor leptin and obesity. Biochem Cell Biol. 1996; 74:615–622. PMID: 9018368.12. Mauvais-Jarvis F. Role of sex steroids in β cell function, growth, and survival. Trends Endocrinol Metab. 2016; 27:844–855. PMID: 27640750.
Article13. Díaz A, López-Grueso R, Gambini J, Monleón D, Mas-Bargues C, Abdelaziz KM, Viña J, Borrás C. Sex differences in age-associated type 2 diabetes in rats-role of estrogens and oxidative stress. Oxid Med Cell Longev. 2019; 2019:6734836. PMID: 31089412.
Article14. Im AR, Ji KY, Park I, Lee JY, Kim KM, Na M, Chae S. Anti-photoaging effects of four insect extracts by downregulating matrix metalloproteinase expression via mitogen-activated protein kinase-dependent signaling. Nutrients. 2019; 11:1159.
Article15. Yoon YI, Chung MY, Hwang JS, Han MS, Goo TW, Yun EY. Allomyrina dichotoma (Arthropoda: Insecta) larvae confer resistance to obesity in mice fed a high-fat diet. Nutrients. 2015; 7:1978–1991. PMID: 25790040.16. Gotoh M, Maki T, Satomi S, Porter J, Bonner-Weir S, O'Hara CJ, Monaco AP. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987; 43:725–730. PMID: 3033857.17. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509. PMID: 13428781.
Article18. Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, Knight AR, Taylor EL, Oettrich J, Ruskovska T, Gasparovic AC, Cuadrado A, Weber D, Poulsen HE, Grune T, Schmidt HH, Ghezzi P. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal. 2015; 23:1144–1170. PMID: 26415143.
Article19. Tamura K, Minami K, Kudo M, Iemoto K, Takahashi H, Seino S. Liraglutide improves pancreatic beta cell mass and function in alloxan-induced diabetic mice. PLoS One. 2015; 10:e0126003. PMID: 25938469.
Article20. Kwon G, Pappan KL, Marshall CA, Schaffer JE, McDaniel ML. cAMP Dose-dependently prevents palmitate-induced apoptosis by both protein kinase A- and cAMP-guanine nucleotide exchange factor-dependent pathways in β-cells. J Biol Chem. 2004; 279:8938–8945. PMID: 14688288.
Article21. Kim JE, Song SE, Kim YW, Kim JY, Park SC, Park YK, Baek SH, Lee IK, Park SY. Adiponectin inhibits palmitate-induced apoptosis through suppression of reactive oxygen species in endothelial cells: involvement of cAMP/protein kinase A and AMP-activated protein kinase. J Endocrinol. 2010; 207:35–44. PMID: 20675307.
Article22. Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto K, Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009; 284:14809–14818. PMID: 19332540.
Article23. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005; 307:384–387. PMID: 15662004.
Article24. Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998; 95:2498–2502. PMID: 9482914.25. Schofield CJ, Sutherland C. Disordered insulin secretion in the development of insulin resistance and type 2 diabetes. Diabet Med. 2012; 29:972–979. PMID: 22443306.26. Ayvaz G, Balos Törüner F, Karakoç A, Yetkin I, Cakir N, Arslan M. Acute and chronic effects of different concentrations of free fatty acids on the insulin secreting function of islets. Diabetes Metab. 2002; 28:3S7–12. PMID: 12688627.27. Fujiwara K, Maekawa F, Yada T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet β-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab. 2005; 289:E670–7. PMID: 15914509.28. Barlow J, Jensen VH, Jastroch M, Affourtit C. Palmitate-induced impairment of glucose-stimulated insulin secretion precedes mitochondrial dysfunction in mouse pancreatic islets. Biochem J. 2016; 473:487–496. PMID: 26621874.
Article29. Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001; 50 Suppl 1:S154–9. PMID: 11272180.
Article30. Tajima K, Shirakawa J, Okuyama T, Kyohara M, Yamazaki S, Togashi Y, Terauchi Y. Effects of metformin on compensatory pancreatic β-cell hyperplasia in mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2017; 313:E367–80. PMID: 28512156.
Article31. Pepin É, Al-Mass A, Attané C, Zhang K, Lamontagne J, Lussier R, Madiraju SR, Joly E, Ruderman NB, Sladek R, Prentki M, Peyot ML. Pancreatic β-cell dysfunction in diet-induced obese mice: roles of AMP-kinase, protein kinase Cε, mitochondrial and cholesterol metabolism, and alterations in gene expression. PLoS One. 2016; 11:e0153017. PMID: 27043434.
Article32. Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006; 147:2643–2649. PMID: 16556766.
Article33. Maechler P, Li N, Casimir M, Vetterli L, Frigerio F, Brun T. Role of mitochondria in beta-cell function and dysfunction. Adv Exp Med Biol. 2010; 654:193–216. PMID: 20217499.34. Shimabukuro M, Ohneda M, Lee Y, Unger RH. Role of nitric oxide in obesity-induced beta cell disease. J Clin Invest. 1997; 100:290–295. PMID: 9218505.
Article35. Salehi A, Carlberg M, Henningson R, Lundquist I. Islet constitutive nitric oxide synthase: biochemical determination and regulatory function. Am J Physiol. 1996; 270:C1634–41. PMID: 8764145.
Article36. Tsuura Y, Ishida H, Shinomura T, Nishimura M, Seino Y. Endogenous nitric oxide inhibits glucose-induced insulin secretion by suppression of phosphofructokinase activity in pancreatic islets. Biochem Biophys Res Commun. 1998; 252:34–38. PMID: 9813142.
Article37. Akesson B, Henningsson R, Salehi A, Lundquist I. Islet constitutive nitric oxide synthase and glucose regulation of insulin release in mice. J Endocrinol. 1999; 163:39–48. PMID: 10495405.
Article38. Kim K, Bae GD, Park EY, Baek DJ, Kim CY, Jang SE, Oh YS. Allomyrina dichotoma larval extract attenuates intestinal barrier disruption by altering inflammatory response and tight junction proteins in lipopolysaccharide-induced Caco-2 cells. Biochem Biophys Res Commun. 2020; 532:145–150. PMID: 32828534.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targets for rescue from fatty acid-induced lipotoxicity in pancreatic beta cells
- The effects of Allomyrina dichotoma larval extract on palmitate-induced insulin resistance in skeletal muscle cells
- Mechanistic insights into pancreatic beta-cell mass regulation by glucose and free fatty acids
- Effects of Free Fatty Acid on Insulin Secretion in Cultured Rat Pancreatic Islets
- Differential Effects of Palmitate and Docosahexaenoic acid on ATP-sensitive K+ Channel Activity of Pancreatic beta-cells