Korean Multicenter Registry Study of EPIC Stents for the Treatment of Iliac Artery Disease: K-EPIC Registry
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Cardiovascular Center, Korea University Guro Hospital, Seoul, Korea
- 3Division of Cardiology, Department of Internal Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- 4Division of Cardiology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoungnam, Korea
- 5Division of Cardiology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Division of Cardiology, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 7Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Chungbuk National University College of Medicine, Korea
- 8Department of Internal Medicine, Cardiovascular Center, Seoul National University Hospital, Seoul, Korea
- 9Cardiology Department, Soonchunhyang University Cheonan Hospital, Cheonan, Korea
- 10Division of Cardiology, Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- KMID: 2515769
- DOI: http://doi.org/10.4070/kcj.2020.0420
Abstract
- Background and Objectives
The EPIC™ stent is a self-expanding, nitinol stent that has been designed to enhance flexibility and provide expansion within vessels. The aim of the present study was to investigate the clinical efficacy and safety of the EPIC™ stent when used to treat iliac artery diseases in a prospective Korean multicenter registry.
Methods
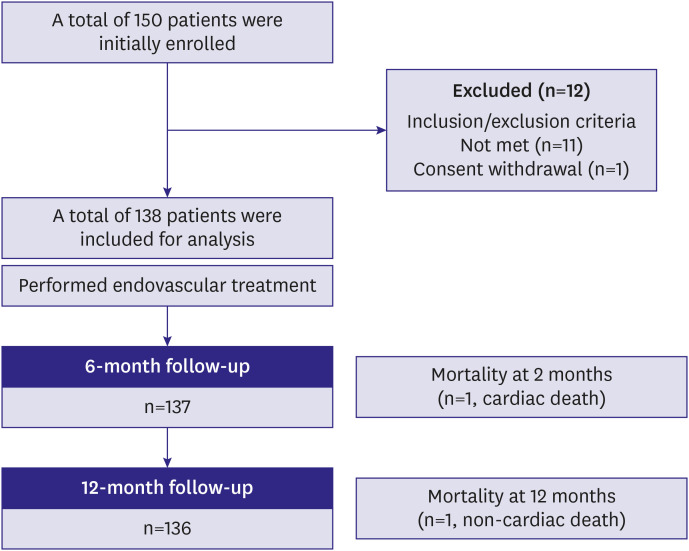
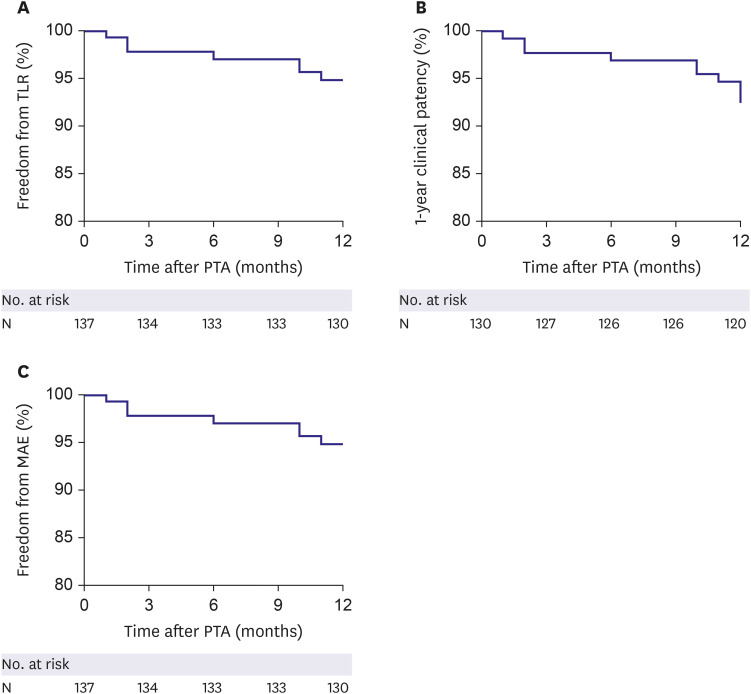
A total of 138 patients with iliac artery diseases who received endovascular treatment with EPIC™ stents at 9 Korean sites were enrolled in a prospective cohort and followed for 1 year. The primary endpoint was the 1-year freedom from target lesion revascularization (TLR). The secondary endpoints were 1-year clinical patency and freedom from major adverse events (MAEs).
Results
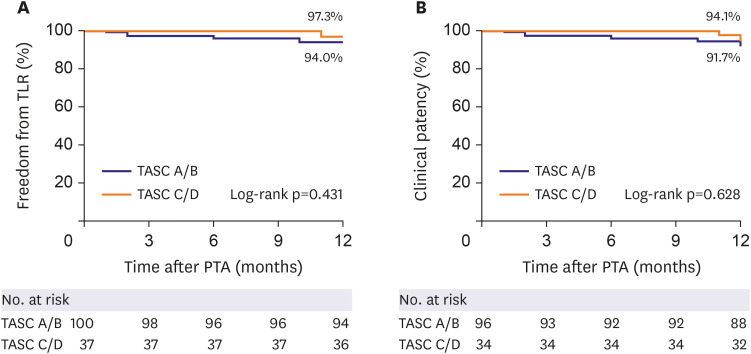
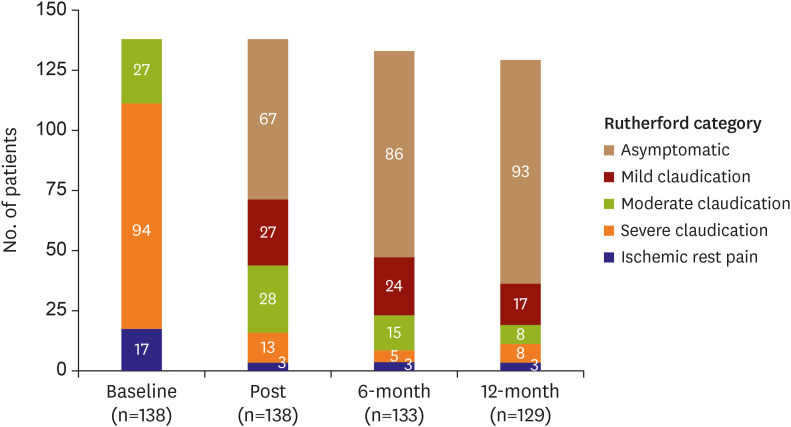
The mean age of the study subjects was 66.8±8.5 years and most subjects were male (86.2%). The most frequent lesion type was Trans-Atlantic Inter-Society Consensus B (43.5%) and the majority (56.5%) of the target lesions were located in the common iliac artery. Procedural success was obtained in 99.3% of patients. The freedom from TLR and the clinical patency at 1-year follow-up were 94.9% and 92.3%, respectively. The 1-year rate of MAEs was 5.1%. Combined coronary artery disease (hazard ratio [HR], 5.91; 95% confidence interval [CI], 1.13–30.89; p=0.035) and smaller stent diameter (HR, 0.38; 95% CI, 0.17–0.88; p=0.023) were identified as independent risk factors of TLR after EPIC™ stent implantation.
Conclusions
The EPIC™ stents demonstrated excellent immediate and 1-year efficacy and safety outcomes in iliac artery lesions in this multicenter, prospective, registry-based study.
Keyword
Figure
Cited by 3 articles
-
Endovascular Therapy of Iliac Artery Disease: Stent Matters
Su Hong Kim
Korean Circ J. 2021;51(5):452-454. doi: 10.4070/kcj.2021.0022.Endovascular Therapy for Complex Iliac Lesions: There Is Much More to Be Defined
Yahya Alansari, Pil Hyung Lee
Korean Circ J. 2022;52(7):541-543. doi: 10.4070/kcj.2022.0145.Long-Term Clinical Outcomes of Iliac Artery Endovascular Therapy in the Korean Vascular Intervention Society Endovascular Therapy in Lower Limb Artery Diseases (K-VIS ELLA) Registry
Ji Woong Roh, Sanghoon Shin, Young-Guk Ko, Nak-Hoon Son, Chul-Min Ahn, Pil-Ki Min, Jae-Hwan Lee, Chang-Hwan Yoon, Cheol Woong Yu, Seung Whan Lee, Sang-Rok Lee, Seung Hyuk Choi, In-Ho Chae, Donghoon Choi
Korean Circ J. 2022;52(7):529-540. doi: 10.4070/kcj.2021.0390.
Reference
-
1. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007; 45(Suppl S):S5–67. PMID: 17223489.
Article2. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017; 69:e71–126. PMID: 27851992.3. Aboyans V, Ricco JB, Bartelink ME, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO). The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018; 39:763–816. PMID: 28886620.4. Ko YG, Ahn CM, Min PK, et al. Baseline characteristics of a retrospective patient cohort in the Korean vascular intervention society endovascular therapy in lower limb artery diseases (K-VIS ELLA) registry. Korean Circ J. 2017; 47:469–476. PMID: 28765738.
Article5. Ye W, Liu CW, Ricco JB, Mani K, Zeng R, Jiang J. Early and late outcomes of percutaneous treatment of TransAtlantic Inter-Society Consensus class C and D aorto-iliac lesions. J Vasc Surg. 2011; 53:1728–1737. PMID: 21609804.
Article6. Kim TI, Schneider PA. Bare metal stenting of the iliac arteries. J Cardiovasc Surg (Torino). 2016; 57:325–335.7. Higashiura W, Kichikawa K, Kubota Y, et al. Does stent affect the configuration of the iliac artery in flexion of the hip joint? Jpn J Endovasc Interv. 2004; 5:22–24.8. Tsujimura T, Iida O, Fujita M, et al. Two-year clinical outcomes post implantation of EpicTM self-expanding nitinol stents for the aortoiliac occlusive disease in patients with peripheral arterial disease. J Atheroscler Thromb. 2018; 25:344–349. PMID: 28978866.
Article9. Lee JH, Cho KI, Spertus J, Kim SM. Cross-cultural adaptation and validation of the Peripheral Artery Questionnaire: Korean version for patients with peripheral vascular diseases. Vasc Med. 2012; 17:215–222. PMID: 22653880.
Article10. Ponec D, Jaff MR, Swischuk J, et al. The Nitinol SMART stent vs Wallstent for suboptimal iliac artery angioplasty: CRISP-US trial results. J Vasc Interv Radiol. 2004; 15:911–918. PMID: 15361558.
Article11. Stoeckel D, Pelton A, Duerig T. Self-expanding nitinol stents: material and design considerations. Eur Radiol. 2004; 14:292–301. PMID: 12955452.
Article12. Burket MW, Brodmann M, Metzger C, Tan K, Jaff MR. Twelve-month results of the nitinol astron stent in iliac artery lesions. J Vasc Interv Radiol. 2016; 27:1650–1656.e1. PMID: 27542591.
Article13. Clair DG, Adams J, Reen B, et al. The EPIC nitinol stent system in the treatment of iliac artery lesions: one-year results from the ORION clinical trial. J Endovasc Ther. 2014; 21:213–222. PMID: 24754280.
Article14. Krankenberg H, Zeller T, Ingwersen M, et al. Self-expanding versus balloon-expandable stents for iliac artery occlusive disease: the randomized ICE trial. JACC Cardiovasc Interv. 2017; 10:1694–1704. PMID: 28838480.15. de Donato G, Bosiers M, Setacci F, et al. 24-month data from the BRAVISSIMO: A large-scale prospective registry on iliac stenting for TASC A & B and TASC C & D lesions. Ann Vasc Surg. 2015; 29:738–750. PMID: 25733220.16. Bechter-Hugl B, Falkensammer J, Gorny O, Greiner A, Chemelli A, Fraedrich G. The influence of gender on patency rates after iliac artery stenting. J Vasc Surg. 2014; 59:1588–1596. PMID: 24548520.
Article17. van Kuijk JP, Flu WJ, Welten GM, et al. Long-term prognosis of patients with peripheral arterial disease with or without polyvascular atherosclerotic disease. Eur Heart J. 2010; 31:992–999. PMID: 20037181.
Article18. Gutierrez JA, Mulder H, Jones WS, et al. Polyvascular disease and risk of major adverse cardiovascular events in peripheral artery disease: a secondary analysis of the EUCLID trial. JAMA Netw Open. 2018; 1:e185239. PMID: 30646395.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early Postoperative Intraperitoneal Chemotherapy for Macroscopically Serosa-Invading Gastric Cancer Patients
- Urinary Incontinence-85: An Expanded Prostate Cancer Composite (EPIC) Score Cutoff Value for Urinary Incontinence Determined Using Long-term Functional Data by Repeated Prospective EPIC-Score Self-assessment After Radical Prostatectomy
- Combined Cytoreductive Surgery and Early Postoperative Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis of Gastric Cancer
- Dose-volume histogram parameters and patient-reported EPIC-Bowel domain in prostate cancer proton therapy
- An experimental study on expandable endovascular metallic stents in dogs