J Korean Neurosurg Soc.
2021 May;64(3):427-436. 10.3340/jkns.2020.0195.
The Fate of Partially Thrombosed Intracranial Aneurysms Treated with Endovascular Intervention
- Affiliations
-
- 1Department of Neurosurgery, Dongguk University Ilsan Hospital, Goyang, Korea
- 2Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea
- 3Department of Radiology, Seoul National University Hospital, Seoul, Korea
- KMID: 2515499
- DOI: http://doi.org/10.3340/jkns.2020.0195
Abstract
Objective
: The fate of partially thrombosed intracranial aneurysms (PTIAs) is not well known after endovascular treatment. The authors aimed to analyze the treatment outcomes of PTIAs.
Methods
: We retrospectively reviewed the medical records of 27 PTIAs treated with endovascular intervention between January 1999 and March 2018. Twenty-one aneurysms were treated with intraluminal embolization (ILE), and six were treated with parent artery occlusion (PAO) with or without bypass surgery. Radiological results, clinical outcomes and risk factors for major recurrence were assessed.
Results
: The initial clinical status was similar in both groups; however, the last status was better in the ILE group than in the PAO group (p=0.049). Neurological deterioration resulted from mass effect in one case and rupture in one after ILE, and mass effect in two and perforator infarction in one after PAO. Twenty cases (94.2%) in the ILE group initially achieved complete occlusion or residual neck status. However, 13 cases (61.9%) showed major recurrence, the major causes of which included coil migration or compaction. Seven cases (33.3%) ultimately achieved residual sac status after repeat treatment. In the PAO group, all initially showed complete occlusion or a residual neck, and just one case ultimately had a residual sac. Two cases showed major recurrence, the cause of which was incomplete PAO. Aneurysm wall calcification was the only significantly protective factor against major recurrence (odds ratio, 36.12; 95% confidence interval, 1.85 to 705.18; p=0.018).
Conclusion
: Complete PAO of PTIAs is the best option if treatment-related complications can be minimized. Simple fluoroscopy is a useful imaging modality because of the recurrence pattern.
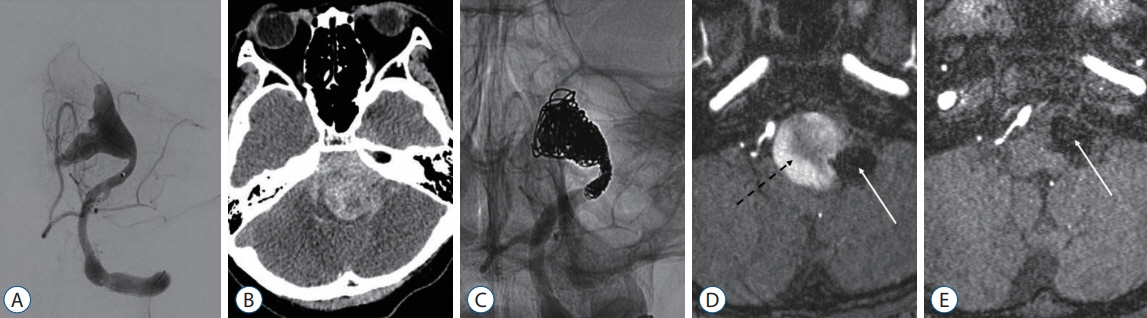
Figure
Reference
-
References
1. Albanese I, Khan K, Barratt B, Al-Kindi H, Schwertani A. Atherosclerotic calcification: Wnt is the hint. J Am Heart Assoc. 7:e007356. 2018.
Article2. Black SP, German WJ. Observations on the relationship between the volume and the size of the orifice of experimental aneurysms. J Neurosurg. 17:984–990. 1960.
Article3. Chalouhi N, Jabbour P, Singhal S, Drueding R, Starke RM, Dalyai RT, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke. 44:1348–1353. 2013.4. Cho WS, Kim JE, Park SQ, Ko JK, Kim DW, Park JC, et al. Korean clinical practice guidelines for aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc. 61:127–166. 2018.
Article5. Cho WS, Lee J, Ha EJ, Kim KH, Lee J, Cho YD, et al. Low-dose prasugrel vs clopidogrel-based tailored premedication for endovascular treatment of cerebral aneurysms. Neurosurgery. 85:E52–E59. 2019.
Article6. Cho YD, Park JC, Kwon BJ, Hee Han M. Endovascular treatment of largely thrombosed saccular aneurysms: follow-up results in ten patients. Neuroradiology. 52:751–758. 2010.
Article7. Doherty TM, Asotra K, Fitzpatrick LA, Qiao JH, Wilkin DJ, Detrano RC, et al. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci USA. 100:11201–11206. 2003.
Article8. Ferns SP, van Rooij WJ, Sluzewski M, van den Berg R, Majoie CB. Partially thrombosed intracranial aneurysms presenting with mass effect: long-term clinical and imaging follow-up after endovascular treatment. AJNR Am J Neuroradiol. 31:1197–1205. 2010.
Article9. Güresir E, Wispel C, Borger V, Hadjiathanasiou A, Vatter H, Schuss P. Treatment of partially thrombosed intracranial aneurysms: single-center series and systematic review. World Neurosurg. 118:e834–e841. 2018.
Article10. Iihara K, Murao K, Sakai N, Soeda A, Ishibashi-Ueda H, Yutani C, et al. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: the role of vasa vasorum. Case report. J Neurosurg. 98:407–413. 2003.
Article11. Kim SJ, Choi IS. Midterm outcome of partially thrombosed intracranial aneurysms treated with guglielmi detachable coils. Interv Neuroradiol. 6:13–25. 2000.
Article12. Krings T, Alvarez H, Reinacher P, Ozanne A, Baccin CE, Gandolfo C, et al. Growth and rupture mechanism of partially thrombosed aneurysms. Interv Neuroradiol. 13:117–126. 2007.
Article13. Lawton MT, Quiñones-Hinojosa A, Chang EF, Yu T. Thrombotic intracranial aneurysms: classification scheme and management strategies in 68 patients. Neurosurgery. 56:441–454. 2005.
Article14. Mizutani T, Miki Y, Kojima H, Suzuki H. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery. 45:253–259. 1999.
Article15. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 360:1267–1274. 2002.
Article16. Nagahiro S, Takada A, Goto S, Kai Y, Ushio Y. Thrombosed growing giant aneurysms of the vertebral artery: growth mechanism and management. J Neurosurg. 82:796–801. 1995.
Article17. Nishido H, Piotin M, Bartolini B, Pistocchi S, Redjem H, Blanc R. Analysis of complications and recurrences of aneurysm coiling with special emphasis on the stent-assisted technique. AJNR Am J Neuroradiol. 35:339–344. 2014.
Article18. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 34:1398–1403. 2003.
Article19. Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J. Longterm follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol. 28:1755–1761. 2007.
Article20. Roccatagliata L, Guédin P, Condette-Auliac S, Gaillard S, Colas F, Boulin A, et al. Partially thrombosed intracranial aneurysms: symptoms, evolution, and therapeutic management. Acta Neurochir (Wien). 152:2133–2142. 2010.
Article21. Scerrati A, Sabatino G, Della Pepa GM, Albanese A, Marchese E, Puca A, et al. Treatment and outcome of thrombosed aneurysms of the middle cerebral artery: institutional experience and a systematic review. Neurosurg Rev. 42:649–661. 2019.
Article22. Schubiger O, Valavanis A, Wichmann W. Growth-mechanism of giant intracranial aneurysms; demonstration by CT and MR imaging. Neuroradiology. 29:266–271. 1987.
Article23. Teng MM, Nasir Qadri SM, Luo CB, Lirng JF, Chen SS, Chang CY. MR imaging of giant intracranial aneurysm. J Clin Neurosci. 10:460–464. 2003.
Article24. Uede T, Ohtaki M, Tanabe S, Hashi K. Direct surgical management of giant and large intracerebral aneurysms, associated with intraluminal thrombus and/or atherosclerotic thickening of aneurysmal neck. No Shinkei Geka. 25:1007–1015. 1997.25. Uyttenboogaart M, Stewart RE, Vroomen PC, De Keyser J, Luijckx GJ. Optimizing cutoff scores for the Barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke. 36:1984–1987. 2005.
Article26. Yang K, Park JC, Ahn JS, Kwon DH, Kwun BD, Kim CJ. Characteristics and outcomes of varied treatment modalities for partially thrombosed intracranial aneurysms: a review of 35 cases. Acta Neurochir (Wien). 156:1669–1675. 2014.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Strategic Dual Approach for the Management of a Symptomatic Giant Partially Thrombosed Aneurysm at the Basilar Tip - Integrating Intrasaccular Flow Diversion and Endovascular Flow Reversal
- Endovascular Treatment of a Large Partially Thrombosed Basilar Tip Aneurysm
- Endovascular treatment of intracranial aneurysms: Past and present
- Guideline for Management of Unruptured Intracranial Aneurysms: Preliminary Report
- Endovascular management of large and giant intracranial aneurysms: Experience from a tertiary care neurosurgery institute in India