Endocrinol Metab.
2021 Apr;36(2):436-446. 10.3803/EnM.2020.883.
High Serum-Induced AhRL Is Associated with Prevalent Metabolic Syndrome and Future Impairment of Glucose Tolerance in the Elderly
- Affiliations
-
- 1Department of Physiology, Kyung Hee University School of Medicine, Graduate School, Seoul, Korea
- 2Department of Neuroscience, Medical Research Center for Bioreaction to Reactive Oxygen Species and Biomedical Science Institute, Kyung Hee University School of Medicine, Graduate School, Seoul, Korea
- 3Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea
- 4Occupational and Environmental Medicine, Uppsala University, Uppsala, Sweden
- 5Acute and Internal Medicine, Department of Medicine, Uppsala University Hospital, Uppsala, Sweden
- 6Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2515469
- DOI: http://doi.org/10.3803/EnM.2020.883
Abstract
- Background
High circulating levels of dioxins and dioxin-like chemicals, acting via the aryl hydrocarbon receptor (AhR), have previously been linked to diabetes. We now investigated whether the serum AhR ligands (AhRL) were higher in subjects with metabolic syndrome (MetS) and in subjects who had developed a worsened glucose tolerance over time.
Methods
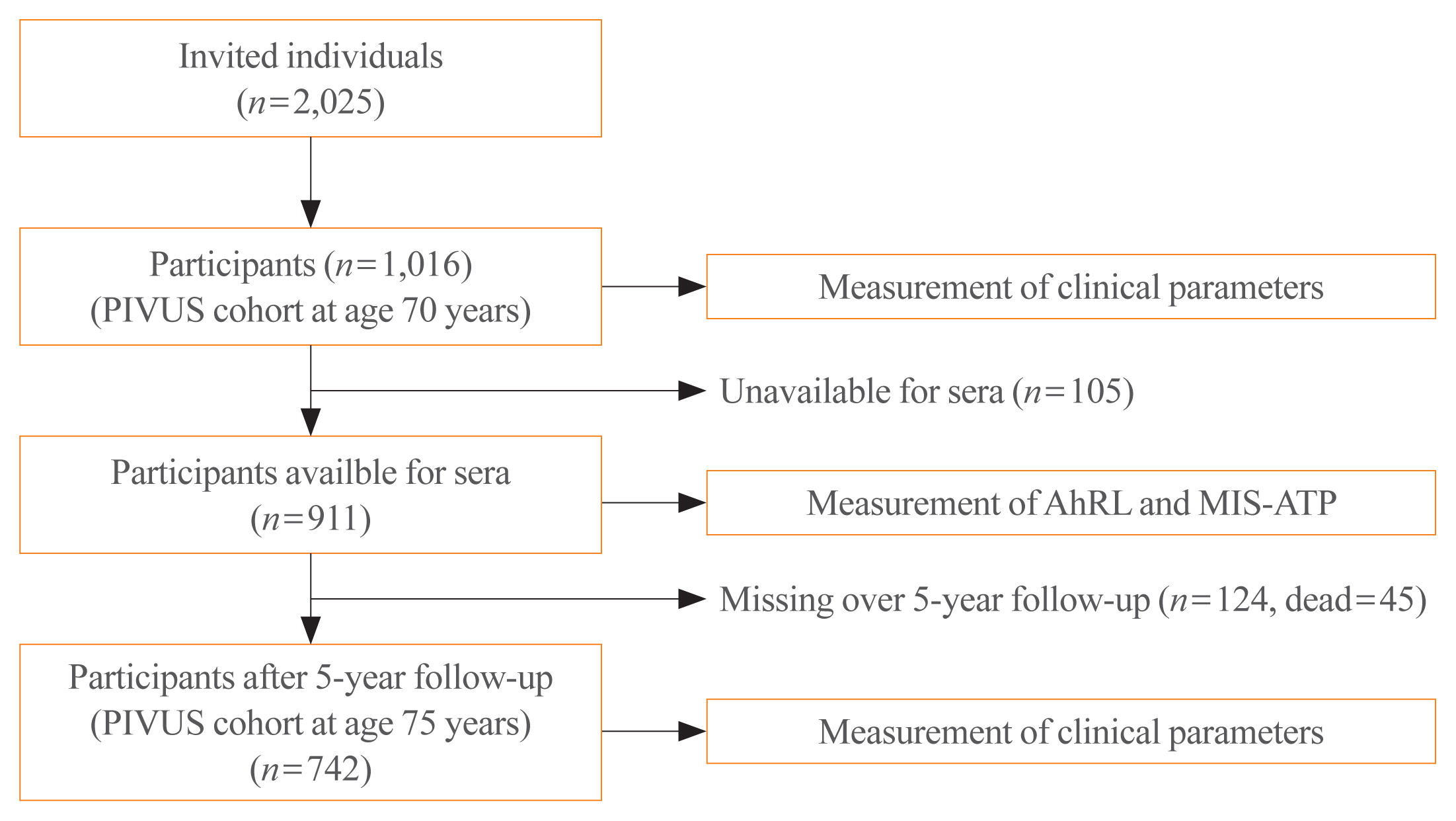
Serum AhRL at baseline was measured by a cell-based AhRL activity assay in 70-year-old subjects (n=911) in the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. The main outcome measures were prevalent MetS and worsening of glucose tolerance over 5 years of follow-up.
Results
AhRL was significantly elevated in subjects with prevalent MetS as compared to those without MetS, following adjustment for sex, smoking, exercise habits, alcohol intake and educational level (P=0.009). AhRL at baseline was higher in subjects who developed impaired fasting glucose or diabetes at age 75 years than in those who remained normoglycemic (P=0.0081). The odds ratio (OR) of AhRL for worsening glucose tolerance over 5 years was 1.43 (95% confidence interval [CI], 1.13 to 1.81; P=0.003, continuous variables) and 2.81 (95% CI, 1.31 to 6.02; P=0.008, in the highest quartile) adjusted for sex, life style factors, body mass index, and glucose.
Conclusion
These findings support a large body of epidemiologic evidence that exposure to AhR transactivating substances, such as dioxins and dioxin-like chemicals, might be involved in the pathogenesis of MetS and diabetes development. Measurement of serum AhRL in humans can be a useful tool in predicting the onset of metabolic disorders.
Keyword
Figure
Reference
-
1. Kylin ES. Studien ueber das hypertonie-hyperglykämie-hyperurikämie syndrome. Zentralblatt fur Innere Medizin. 1923; 44:105–27.2. Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. 2008; 29:777–822.
Article3. Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes. 1988; 37:1595–607.
Article4. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–53.
Article5. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015; 36:E1–150.
Article6. World Health Organization-United Nations Environment Programme (WHO-UNEP). State of the science of endocrine disrupting chemicals 2012 summary for decision-makers [Internet]. Geneva: WHO;2013. [cited 2021 Mar 22]. Available from: http://www.who.int/ceh/publications/endocrine/en/index.html.7. Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007; 30:622–8.8. Lind PM, Riserus U, Salihovic S, Bavel Bv, Lind L. An environmental wide association study (EWAS) approach to the metabolic syndrome. Environ Int. 2013; 55:1–8.
Article9. Uemura H, Arisawa K, Hiyoshi M, Kitayama A, Takami H, Sawachika F, et al. Prevalence of metabolic syndrome associated with body burden levels of dioxin and related compounds among Japan’s general population. Environ Health Perspect. 2009; 117:568–73.
Article10. Lee DH, Lind L, Jacobs DR Jr, Salihovic S, van Bavel B, Lind PM. Associations of persistent organic pollutants with abdominal obesity in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Environ Int. 2012; 40:170–8.
Article11. Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006; 29:1638–44.12. Magliano DJ, Loh VH, Harding JL, Botton J, Shaw JE. Persistent organic pollutants and diabetes: a review of the epidemiological evidence. Diabetes Metab. 2014; 40:1–14.
Article13. Lind L, Zethelius B, Salihovic S, van Bavel B, Lind PM. Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia. 2014; 57:473–9.
Article14. Lind L, Lind PM, Lejonklou MH, Dunder L, Bergman A, Guerrero-Bosagna C, et al. Uppsala consensus statement on environmental contaminants and the global obesity epidemic. Environ Health Perspect. 2016; 124:A81–3.
Article15. Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013; 121:774–83.
Article16. Lind PM, Lind L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia. 2018; 61:1495–502.
Article17. Warner M, Mocarelli P, Brambilla P, Wesselink A, Samuels S, Signorini S, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso women’s health study. Environ Health Perspect. 2013; 121:906–11.
Article18. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. Executive summary to EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015; 36:593–602.
Article19. Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol. 2005; 25:2368–75.20. Park WH, Jun DW, Kim JT, Jeong JH, Park H, Chang YS, et al. Novel cell-based assay reveals associations of circulating serum AhR-ligands with metabolic syndrome and mitochondrial dysfunction. Biofactors. 2013; 39:494–504.
Article21. Park WH, Kang S, Lee HK, Salihovic S, Bavel BV, Lind PM, et al. Relationships between serum-induced AhR bioactivity or mitochondrial inhibition and circulating polychlorinated biphenyls (PCBs). Sci Rep. 2017; 7:9383.
Article22. Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008; 18:207–50.
Article23. Hao N, Whitelaw ML. The emerging roles of AhR in physiology and immunity. Biochem Pharmacol. 2013; 86:561–70.
Article24. Okey AB. An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology-XI. Toxicol Sci. 2007; 98:5–38.
Article25. Brennan JC, He G, Tsutsumi T, Zhao J, Wirth E, Fulton MH, et al. Development of species-specific ah receptor-responsive third generation CALUX cell lines with enhanced responsiveness and improved detection limits. Environ Sci Technol. 2015; 49:11903–12.
Article26. Lee HK, Park WH, Kang YC, Kang S, Im S, Park S, et al. Serum biomarkers from cell-based assays for AhRL and MIS strongly predicted the future development of diabetes in a large community-based prospective study in Korea. Sci Rep. 2020; 10:6339.
Article27. Lee DH, Lind PM, Jacobs DR Jr, Salihovic S, van Bavel B, Lind L. Association between background exposure to organochlorine pesticides and the risk of cognitive impairment: a prospective study that accounts for weight change. Environ Int. 2016; 89–90:179–84.
Article28. Kim JT, Kim SS, Jun DW, Hwang YH, Park WH, Pak YK, et al. Serum arylhydrocarbon receptor transactivating activity is elevated in type 2 diabetic patients with diabetic nephropathy. J Diabetes Investig. 2013; 4:483–91.
Article29. Piao Y, Kim HG, Oh MS, Pak YK. Overexpression of TFAM, NRF-1 and myr-AKT protects the MPP(+)-induced mitochondrial dysfunctions in neuronal cells. Biochim Biophys Acta. 2012; 1820:577–85.
Article30. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–97.31. Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995; 35:307–40.
Article32. Olsman H, Engwall M, Kammann U, Klempt M, Otte J, van Bavel B, et al. Relative differences in aryl hydrocarbon receptor-mediated response for 18 polybrominated and mixed halogenated dibenzo-p-dioxins and -furans in cell lines from four different species. Environ Toxicol Chem. 2007; 26:2448–54.
Article33. Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011; 334:1561–5.
Article34. Doan TQ, Berntsen HF, Verhaegen S, Ropstad E, Connolly L, Igout A, et al. A mixture of persistent organic pollutants relevant for human exposure inhibits the transactivation activity of the aryl hydrocarbon receptor in vitro. Environ Pollut. 2019; 254(Pt B):113098.35. Lim S, Ahn SY, Song IC, Chung MH, Jang HC, Park KS, et al. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One. 2009; 4:e5186.
Article36. Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010; 118:465–71.
Article37. Eichbaum K, Brinkmann M, Buchinger S, Reifferscheid G, Hecker M, Giesy JP, et al. In vitro bioassays for detecting dioxin-like activity: application potentials and limits of detection, a review. Sci Total Environ. 2014; 487:37–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prevalence of Metabolic Syndrome according to the Degree of Glucose Metabolism Impairment
- Correlation of C-reactive Protein with Components of Metabolic Syndrome in Elderly Korean Women with Normal or Impaired Glucose Tolerance
- Peripheral Neuropathy in Metabolic Syndrome
- Relationship between Serum Adiponectin and Development of the Metabolic Syndrome
- Relationships between Insulin-like Growth Factor-I and Severity of Hepatic Dysfunction, and Glucose Tolerance Status in Patients with Liver Cirrhosis