Clin Exp Otorhinolaryngol.
2021 May;14(2):217-224. 10.21053/ceo.2020.00444.
Human Rhinovirus Infection Enhances the Th2 Environment in Allergic and Non-allergic Patients with Chronic Rhinosinusitis
- Affiliations
-
- 1Graduate School of Medicine, Seoul National University College of Medicine, Seoul, Korea
- 2Center of Morphological Experiment, Medical College of Yanbian University, Yanji, China
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 6Sensory Organs Research Center, Seoul National University Medical Research Center, Seoul, Korea
- 7Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul, Korea
- KMID: 2515435
- DOI: http://doi.org/10.21053/ceo.2020.00444
Abstract
Objectives
. This study was conducted to determine whether patients with allergic rhinitis might be more susceptible to human rhinovirus (HRV) infection and whether the effects of infection on the elicited immune responses are different in allergic and non-allergic patients with chronic rhinosinusitis (CRS).
Methods
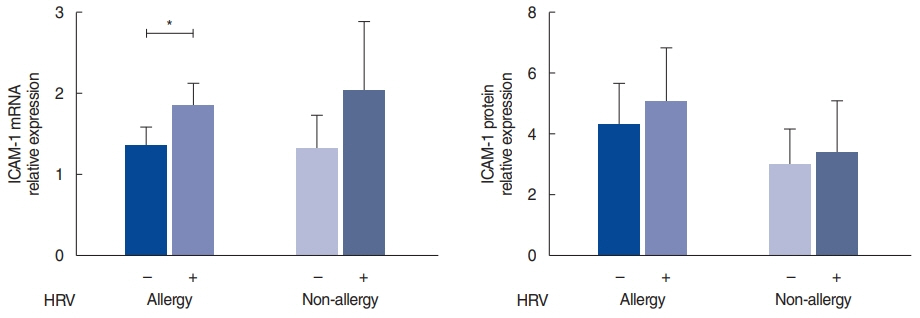
. Uncinate process tissues were obtained from 61 CRS patients (of whom 39 had allergies and 22 did not) and were infected with HRV-16 using an air-liquid interface organ culture system. The expression levels of programmed cell death-ligand (PD-L)1, PD-L2, intracellular adhesion molecule 1, interferon-gamma (IFN-γ), interleukin (IL)-4, IL-5, and IL-10 were evaluated in the infected nasal mucosa.
Results
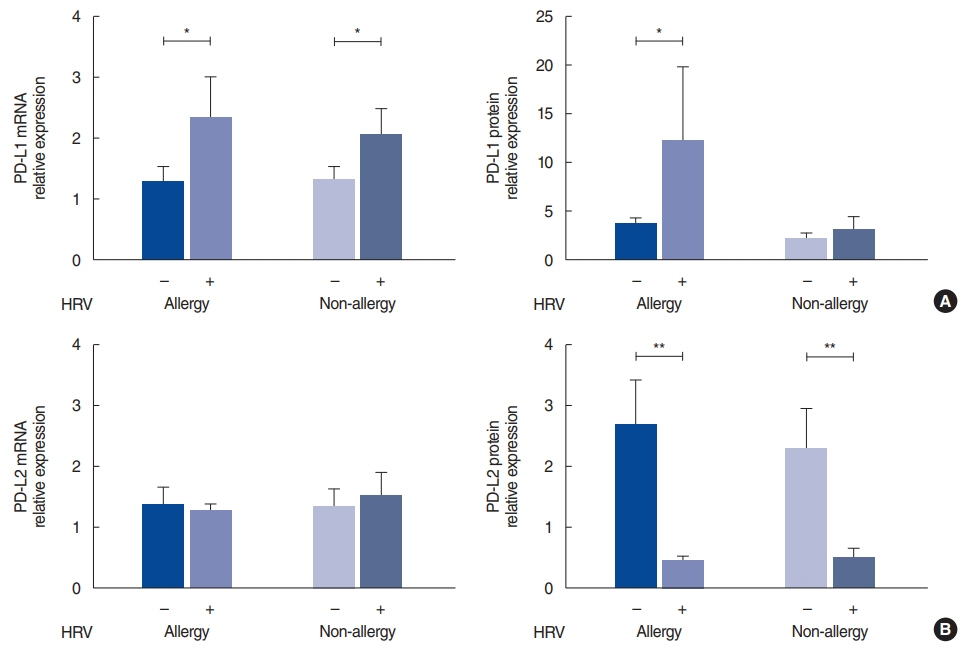
. The HRV infection rates were not significantly different between the allergy (74.4%) and non-allergy (72.7%) groups. In the allergy group, the expression of PD-L1 (P=0.013) and IL-10 (P=0.040) was significantly elevated in the HRV-infected tissues, and there was a strong correlation between PD-L1 and IL-10 (r=0.868, P<0.001). In contrast, infected tissues from the non-allergy group displayed increased levels of IL-4 (P=0.039), IL-5 (P=0.023), and IFN-γ (P=0.031), as well as an increased IL-4/IFN-γ ratio, after HRV infection (P=0.043).
Conclusion
. This study showed that HRV infection rates were similar in the nasal mucosa of patients with CRS regardless of the presence of allergic rhinitis. HRV infection enhanced the Th2 environment by modulating PD-L1 and PD-L2 expression levels in allergic mucosa and by increasing the IL-4/IFN-γ ratio in non-allergic mucosa.
Figure
Reference
-
1. Jacobs SE, Lamson DM, St George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev. 2013; Jan. 26(1):135–62.
Article2. Pomeranz G, Pando R, Hindiyeh M, Sherbany H, Meningher T, Sharabi S, et al. Rhinovirus infections in infants suggest that early detection can prevent unnecessary treatment. J Clin Virol. 2019; Jun. 115:11–7.
Article3. Drysdale SB, Mejias A, Ramilo O. Rhinovirus: not just the common cold. J Infect. 2017; Jun. 74 Suppl 1:S41–6.4. Proud D. Role of rhinovirus infections in asthma. Asian Pac J Allergy Immunol. 2011; Sep. 29(3):201–8.5. Juhn YJ. Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease. J Allergy Clin Immunol. 2014; Aug. 134(2):247–57.
Article6. Singh AK, Stock P, Akbari O. Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy. 2011; Feb. 66(2):155–62.
Article7. Kortekaas Krohn I, Bobic S, Dooley J, Lan F, Zhang N, Bachert C, et al. Programmed cell death-1 expression correlates with disease severity and IL-5 in chronic rhinosinusitis with nasal polyps. Allergy. 2017; Jun. 72(6):985–93.
Article8. Deppong C, Juehne TI, Hurchla M, Friend LD, Shah DD, Rose CM, et al. Cutting edge: B and T lymphocyte attenuator and programmed death receptor-1 inhibitory receptors are required for termination of acute allergic airway inflammation. J Immunol. 2006; Apr. 176(7):3909–13.
Article9. Zdrenghea MT, Johnston SL. Role of PD-L1/PD-1 in the immune response to respiratory viral infections. Microbes Infect. 2012; Jun. 14(6):495–9.
Article10. Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010; Jan. 3(1):81–91.
Article11. Ishiwata K, Watanabe N, Guo M, Tomihara K, Brumlik MJ, Yagita H, et al. Costimulator B7-DC attenuates strong Th2 responses induced by Nippostrongylus brasiliensis. J Immunol. 2010; Feb. 184(4):2086–94.
Article12. Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010; Apr. 16(4):452–9.
Article13. Kosaka S, Tamauchi H, Terashima M, Maruyama H, Habu S, Kitasato H. IL-10 controls Th2-type cytokine production and eosinophil infiltration in a mouse model of allergic airway inflammation. Immunobiology. 2011; Jul. 216(7):811–20.
Article14. Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun. 2015; Jan. 6:5997.
Article15. Koya T, Matsuda H, Takeda K, Matsubara S, Miyahara N, Balhorn A, et al. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J Allergy Clin Immunol. 2007; May. 119(5):1241–50.
Article16. Wang JH, Kwon HJ, Chung YS, Lee BJ, Jang YJ. Infection rate and virus-induced cytokine secretion in experimental rhinovirus infection in mucosal organ culture: comparison between specimens from patients with chronic rhinosinusitis with nasal polyps and those from normal subjects. Arch Otolaryngol Head Neck Surg. 2008; Apr. 134(4):424–7.17. Rhee CS, Min YG, Lee CH, Kwon TY, Lee CH, Yi WJ, et al. Ciliary beat frequency in cultured human nasal epithelial cells. Ann Otol Rhinol Laryngol. 2001; Nov. 110(11):1011–6.
Article18. Kloepfer KM, Olenec JP, Lee WM, Liu G, Vrtis RF, Roberg KA, et al. Increased H1N1 infection rate in children with asthma. Am J Respir Crit Care Med. 2012; Jun. 185(12):1275–9.
Article19. Santillan Salas CF, Mehra S, Pardo Crespo MR, Juhn YJ. Asthma and severity of 2009 novel H1N1 influenza: a population-based case-control study. J Asthma. 2013; Dec. 50(10):1069–76.
Article20. Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med. 2012; May. 18(5):726–35.
Article21. Caliskan M, Bochkov YA, Kreiner-Moller E, Bonnelykke K, Stein MM, Du G, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013; Apr. 368(15):1398–407.
Article22. DeMore JP, Weisshaar EH, Vrtis RF, Swenson CA, Evans MD, Morin A, et al. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J Allergy Clin Immunol. 2009; Aug. 124(2):245–52.
Article23. Kim WK, Gern JE. Updates in the relationship between human rhinovirus and asthma. Allergy Asthma Immunol Res. 2012; May. 4(3):116–21.
Article24. Matsumoto K, Fukuyama S, Eguchi-Tsuda M, Nakano T, Matsumoto T, Matsumura M, et al. B7-DC induced by IL-13 works as a feedback regulator in the effector phase of allergic asthma. Biochem Biophys Res Commun. 2008; Jan. 365(1):170–5.
Article25. Matsumoto K, Kan-O K, Eguchi-Tsuda M, Fukuyama S, Asai Y, Matsumoto T, et al. Essential role of B7-H1 in double-stranded RNA-induced augmentation of an asthma phenotype in mice. Am J Respir Cell Mol Biol. 2011; Jul. 45(1):31–9.
Article26. Herbert C, Do K, Chiu V, Garthwaite L, Chen Y, Young PM, et al. Allergic environment enhances airway epithelial pro-inflammatory responses to rhinovirus infection. Clin Sci (Lond). 2017; Mar. 131(6):499–509.
Article27. Oyer SL, Nagel W, Mulligan JK. Differential expression of adhesion molecules by sinonasal fibroblasts among control and chronic rhinosinusitis patients. Am J Rhinol Allergy. 2013; Sep-Oct. 27(5):381–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Allergy in the Severity of Chronic Rhinosinusitis
- Microbiome of the upper airway focusing on chronic rhinosinusitis and allergic rhinitis
- Extra-Intestinal Manifestation of Helicobacter pylori Infection: Allergic Diseases Including Asthma, Atopy and Idiopathic Urticaria
- Vitamin D and Allergic Disease
- Increase of Rhinovirus Replication in Airway Epithelial Cells by Staphylococcal Enterotoxin A and B