Cancer Res Treat.
2021 Apr;53(2):457-470. 10.4143/crt.2020.585.
Effect of Combining EGFR Tyrosine Kinase Inhibitors and Cytotoxic Agents on Cholangiocarcinoma Cells
- Affiliations
-
- 1Department of Anatomy, Faculty of Science, Mahidol University, Bangkok, Thailand
- 2Division of Cancer and Stem Cells, Centre for Cancer Sciences, School of Medicine, Biodiscovery Institute, University of Nottingham, Nottingham, UK
- 3Department of Biochemistry, Faculty of Science, Mahidol University, Bangkok, Thailand
- 4Department of Anatomy, Faculty of Medicine, Siam University, Bangkok, Thailand
- 5Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 6Molecular Medicine Program, Multidisciplinary Unit, Faculty of Science, Mahidol University, Bangkok, Thailand
- 7Department of Cellular Pathology, Queen’s Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham, UK
- 8Department of Hepatobiliary and Pancreatic Surgery, and NIHR Nottingham Digestive Disease Biomedical Research Unit, University of Nottingham, Nottingham, UK
- 9Department of Biology, Faculty of Science, Mahidol University, Bangkok, Thailand
- 10Department of Pathology, Rajavithi Hospital, Bangkok, Thailand
- KMID: 2514927
- DOI: http://doi.org/10.4143/crt.2020.585
Abstract
- Purpose
The potential of members of the epidermal growth factor receptor (ErbB) family as drug targets in cholangiocarcinoma (CCA) has not been extensively addressed. Although phase III clinical trials showed no survival benefits of erlotinib in patients with advanced CCA, the outcome of the standard-of-care chemotherapy treatment for CCA, gemcitabine/cisplatin, is discouraging so we determined the effect of other ErbB receptor inhibitors alone or in conjunction with chemotherapy in CCA cells. Materials and Methods ErbB receptor expression was determined in CCA patient tissues by immunohistochemistry and digital-droplet polymerase chain reaction, and in primary cells and cell lines by immunoblot. Effects on cell viability and cell cycle distribution of combination therapy using ErbB inhibitors with chemotherapeutic drugs was carried out in CCA cell lines. 3D culture of primary CCA cells was then adopted to evaluate the drug effect in a setting that more closely resembles in vivo cell environments.
Results
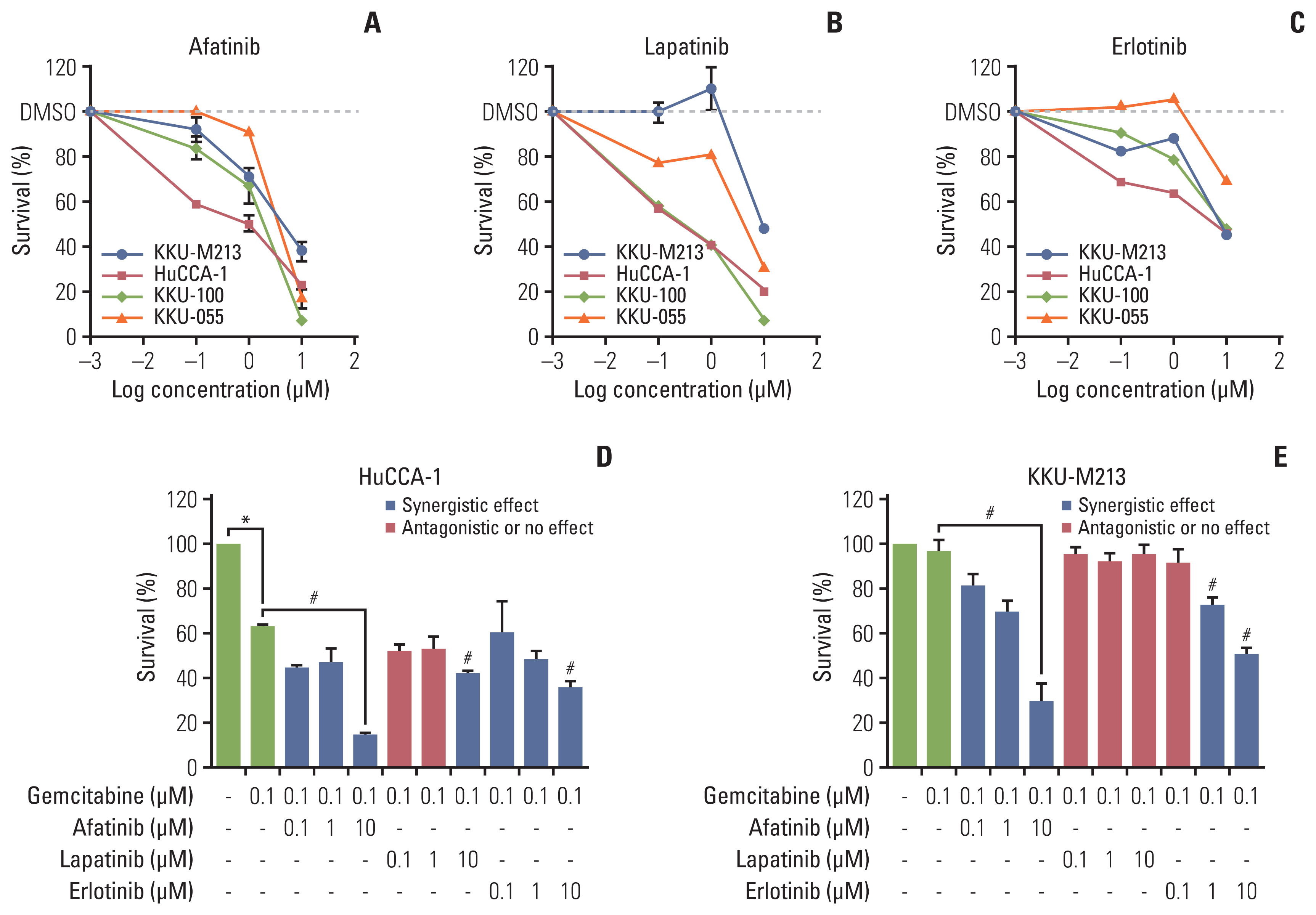
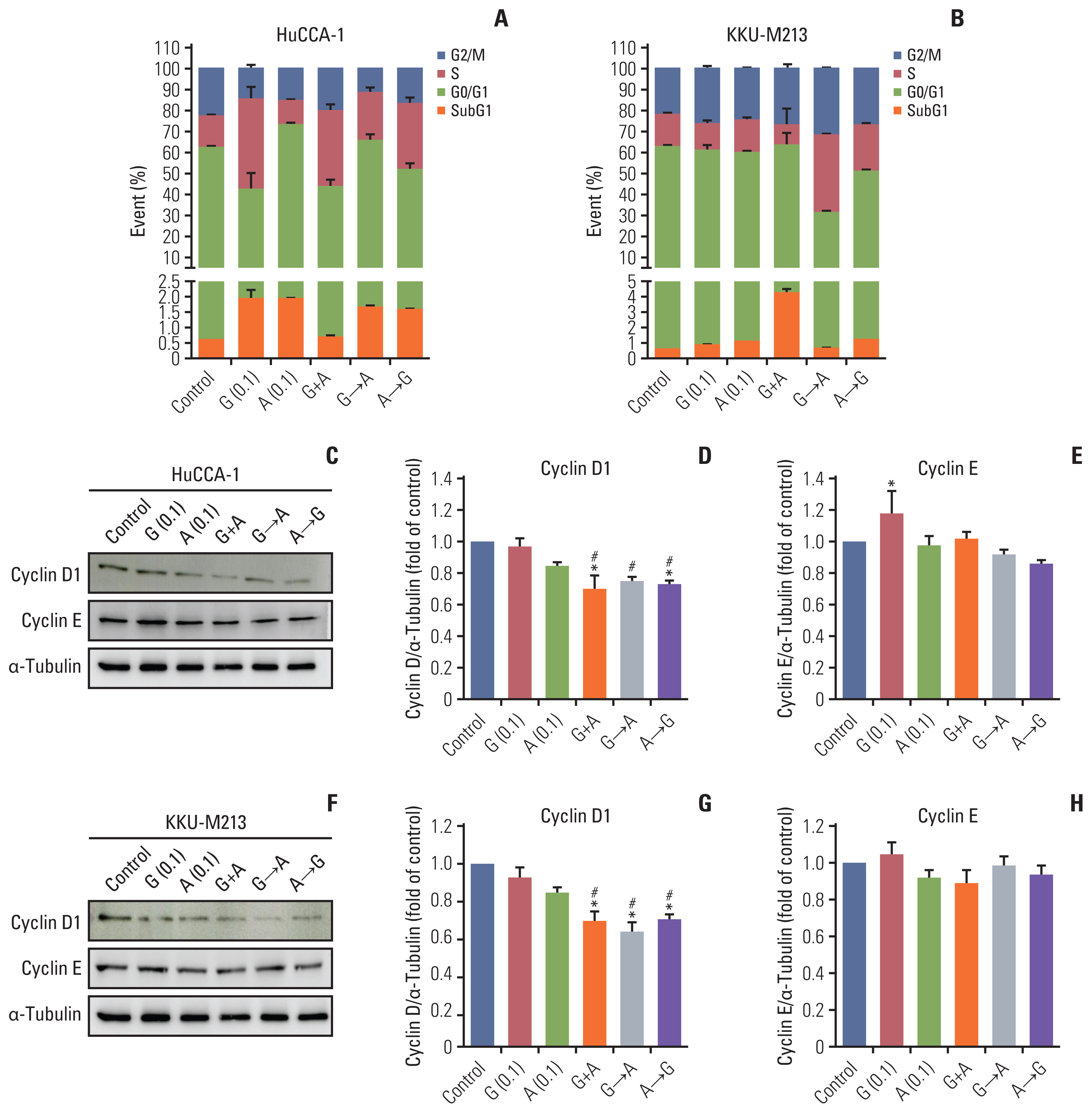
CCA tumors showed higher expression of all ErbB receptors compared with resection margins. Primary and CCA cell lines had variable expression of erbB receptors. CCA cell lines showed decreased cell viability when treated with chemotherapeutic drugs (gemcitabine and 5-fluorouracil) but also with ErbB inhibitors, particularly afatinib, and with a combination. Sequential treatment of gemcitabine with afatinib was particularly effective. Co-culture of CCA primary cells with cancer-associated fibroblasts decreased sensitivity to chemotherapies, but sensitized to afatinib. Conclusion Afatinib is a potential epidermal growth factor receptor targeted drug for CCA treatment and sequential treatment schedule of gemcitabine and afatinib could be explored in CCA patients.
Keyword
Figure
Reference
-
References
1. Konfortion J, Jack RH, Davies EA. Coverage of common cancer types in UK national newspapers: a content analysis. BMJ Open. 2014; 4:e004677.
Article2. Maroni L, Pierantonelli I, Banales JM, Benedetti A, Marzioni M. The significance of genetics for cholangiocarcinoma development. Ann Transl Med. 2013; 1:28.3. Sahu S, Sun W. Targeted therapy in biliary tract cancers-current limitations and potentials in the future. J Gastrointest Oncol. 2017; 8:324–36.
Article4. Hyung J, Kim B, Yoo C, Kim KP, Jeong JH, Chang HM, et al. Clinical benefit of maintenance therapy for advanced biliary tract cancer patients showing no progression after first-line gemcitabine plus cisplatin. Cancer Res Treat. 2019; 51:901–9.
Article5. Ritter CA, Arteaga CL. The epidermal growth factor receptor-tyrosine kinase: a promising therapeutic target in solid tumors. Semin Oncol. 2003; 30(1 Suppl 1):3–11.
Article6. Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001; 7:2958–70.7. Roskoski R Jr. ErbB/HER protein-tyrosine kinases: Structures and small molecule inhibitors. Pharmacol Res. 2014; 87:42–59.
Article8. Moehler M, Maderer A, Ehrlich A, Foerster F, Schad A, Nickolay T, et al. Safety and efficacy of afatinib as add-on to standard therapy of gemcitabine/cisplatin in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, phase I trial with an extensive biomarker program. BMC Cancer. 2019; 19:55.
Article9. Peck J, Wei L, Zalupski M, O’Neil B, Villalona Calero M, Bekaii-Saab T. HER2/neu may not be an interesting target in biliary cancers: results of an early phase II study with lapatinib. Oncology. 2012; 82:175–9.
Article10. Pellat A, Vaquero J, Fouassier L. Role of ErbB/HER family of receptor tyrosine kinases in cholangiocyte biology. Hepatology. 2018; 67:762–73.
Article11. Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003; 21:3798–807.
Article12. Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012; 25:637–50.
Article13. Bartley AN, Washington MK, Ventura CB, Ismaila N, Colasacco C, Benson AB 3rd, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Am J Clin Pathol. 2016; 146:647–69.
Article14. Sirisinha S, Tengchaisri T, Boonpucknavig S, Prempracha N, Ratanarapee S, Pausawasdi A. Establishment and characterization of a cholangiocarcinoma cell line from a Thai patient with intrahepatic bile duct cancer. Asian Pac J Allergy Immunol. 1991; 9:153–7.15. Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, et al. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol. 2005; 11:3392–7.
Article16. Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012; 180:599–607.
Article17. Saunders JH, Onion D, Collier P, Dorrington MS, Argent RH, Clarke PA, et al. Individual patient oesophageal cancer 3D models for tailored treatment. Oncotarget. 2017; 8:24224–36.
Article18. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010; 70:440–6.
Article19. Zografos GN, Farfaras A, Zagouri F, Chrysikos D, Karaliotas K. Cholangiocarcinoma: principles and current trends. Hepatobiliary Pancreat Dis Int. 2011; 10:10–20.
Article20. Yang X, Wang W, Wang C, Wang L, Yang M, Qi M, et al. Characterization of EGFR family gene aberrations in cholangiocarcinoma. Oncol Rep. 2014; 32:700–8.
Article21. Komoto M, Nakata B, Nishii T, Kawajiri H, Shinto O, Amano R, et al. In vitro and in vivo evidence that a combination of lapatinib plus S-1 is a promising treatment for pancreatic cancer. Cancer Sci. 2010; 101:468–73.22. Yokoyama M, Ohnishi H, Ohtsuka K, Matsushima S, Ohkura Y, Furuse J, et al. KRAS mutation as a potential prognostic biomarker of biliary tract cancers. Jpn Clin Med. 2016; 7:33–9.
Article23. Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012; 486:532–6.
Article24. Canale M, Petracci E, Delmonte A, Chiadini E, Dazzi C, Papi M, et al. Impact of TP53 mutations on outcome in EGFR-mutated patients treated with first-line tyrosine kinase inhibitors. Clin Cancer Res. 2017; 23:2195–202.
Article25. Royds JA, Iacopetta B. p53 and disease: when the guardian angel fails. Cell Death Differ. 2006; 13:1017–26.
Article26. Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017; 8:8921–46.
Article27. Kim Y, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Efficacy and safety of afatinib for EGFR-mutant non-small cell lung cancer, compared with gefitinib or erlotinib. Cancer Res Treat. 2019; 51:502–9.
Article28. Lu X, Liu J, Legerski RJ. Cyclin E is stabilized in response to replication fork barriers leading to prolonged S phase arrest. J Biol Chem. 2009; 284:35325–37.
Article29. Li T, Ling YH, Goldman ID, Perez-Soler R. Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cells. Clin Cancer Res. 2007; 13:3413–22.
Article30. Ubezio P, Falcetta F, Carrassa L, Lupi M. Integrated experimental and simulation study of the response to sequential treatment with erlotinib and gemcitabine in pancreatic cancer. Oncotarget. 2016; 7:15492–506.
Article31. Xiang T, Li L, Yin X, Yuan C, Tan C, Su X, et al. The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer. PLoS One. 2012; 7:e29783.
Article32. Kitchen P, Lee KY, Clark D, Lau N, Lertsuwan J, Sawasdichai A, et al. A runaway PRH/HHEX-Notch3-positive feedback loop drives cholangiocarcinoma and determines response to CDK4/6 inhibition. Cancer Res. 2020; 80:757–70.
Article33. Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009; 21:177–84.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Basis of Drug Resistance: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors and Anaplastic Lymphoma Kinase Inhibitors
- EGFR Tyrosine Kinase Inhibitors for NSCLC
- Chronicles of EGFR Tyrosine Kinase Inhibitors: Targeting EGFR C797S Containing Triple Mutations
- Combining Erlotinib with Cytotoxic Chemotherapy May Overcome Resistance Caused by T790M Mutation of EGFR Gene in Non-Small Cell Lung Carcinoma
- Targeted therapy and tailored chemotherapy for advanced non-small cell lung cancer