J Korean Med Sci.
2021 Apr;36(14):e90. 10.3346/jkms.2021.36.e90.
Increased Expression of S100B and RAGE in a Mouse Model of Bile Duct Ligation-induced Liver Fibrosis
- Affiliations
-
- 1Department of Biomedical Gerontology, Graduate School of Hallym University, Chuncheon, Korea
- 2Department of Internal Medicine, Hallym University Medical Center, Anyang, Korea
- 3Institute for Liver and Digestive Diseases, Hallym University, Chuncheon, Korea
- 4Ilsong Institute of Life Science, Hallym University, Anyang, Korea
- 5Department of Internal Medicine, Kangdong Sacred Heart Hospital of Hallym University Medical Center, Seoul, Korea
- 6Department of Internal Medicine, Chuncheon Sacred Heart Hospital of Hallym University Medical Center, Chuncheon, Korea
- KMID: 2514901
- DOI: http://doi.org/10.3346/jkms.2021.36.e90
Abstract
- Background
Liver fibrosis is defined as the accumulation of the extracellular matrix and scar formation. The receptor for advanced glycation end products (RAGE) has been demonstrated to participate in fibrogenesis. S100B is a ligand of RAGE and exerts extracellular functions by inducing a series of signal transduction cascades. However, the involvement of S100B and RAGE in cholestasis-induced liver fibrosis remains unclear. In this study, we investigated S100B and RAGE expression during liver fibrosis in mice that underwent common bile duct ligation (BDL).
Methods
BDL was performed in 10-week-old male C57BL/6J mice with sham control (n = 26) and BDL (n = 26) groups. Expression levels of S100B, RAGE and fibrotic markers in the livers from both groups at week 1 and 3 after BDL were examined by western blot and quantitative real-time reverse transcription polymerase chain reaction analysis. Liver fibrotic changes were examined by histological and ultrastructural analysis.
Results
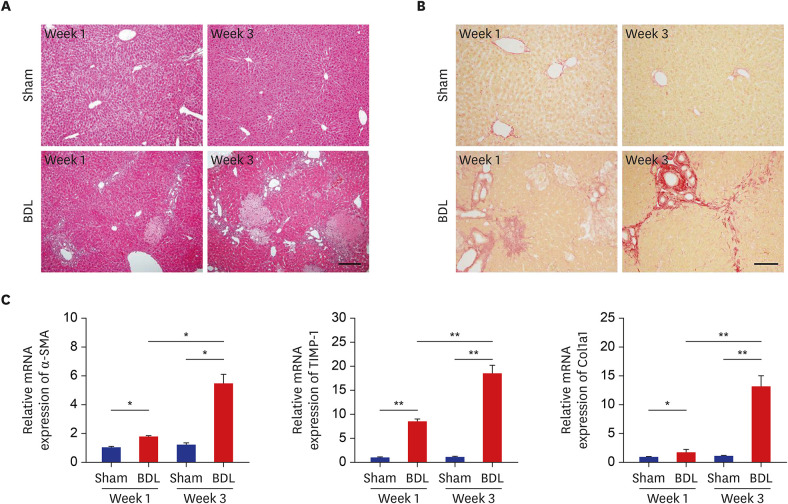
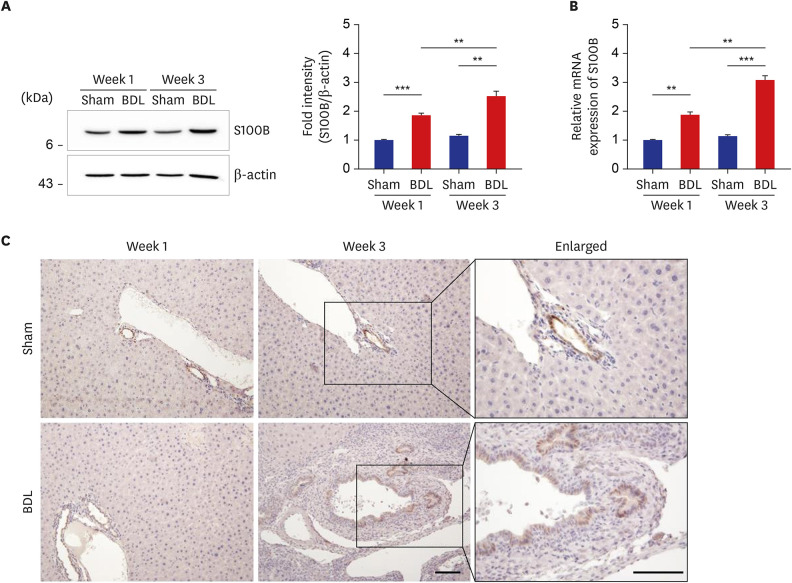
Histological staining with Sirius Red and the evaluation of the messenger RNA expression of fibrotic markers showed noticeable periportal fibrosis and bile duct proliferation. S100B was mainly present in bile duct epithelial cells, and its expression was upregulated in proportion to the ductular reaction during fibrogenesis by BDL. RAGE expression was also increased, and interestingly, triple immunofluorescence staining and transmission electron microscopy showed that both S100B and RAGE were expressed in proliferating bile duct epithelial cells and activated hepatic stellate cells (HSCs) of the BDL livers. In addition, in rat HSCs (HSC-T6), treatment with recombinant S100B protein significantly increased fibrotic markers in a dose-dependent manner, and RAGE small interfering RNA (siRNA) suppressed S100B-stimulated upregulation of fibrotic markers compared with cells treated with scramble siRNA and S100B.
Conclusion
These findings suggest that the increased expression of S100B and RAGE and the interaction between S100B and RAGE may play an important role in ductular reaction and liver fibrosis induced by BDL.
Figure
Reference
-
1. Tacke F, Trautwein C. Mechanisms of liver fibrosis resolution. J Hepatol. 2015; 63(4):1038–1039. PMID: 26232376.
Article2. Pinzani M. Pathophysiology of liver fibrosis. Dig Dis. 2015; 33(4):492–497. PMID: 26159264.
Article3. Lemoinne S, Cadoret A, El Mourabit H, Thabut D, Housset C. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013; 1832(7):948–954. PMID: 23470555.
Article4. Xia JR, Liu NF, Zhu NX. Specific siRNA targeting the receptor for advanced glycation end products inhibits experimental hepatic fibrosis in rats. Int J Mol Sci. 2008; 9(4):638–661. PMID: 19325776.
Article5. Yan SF, Yan SD, Ramasamy R, Schmidt AM. Tempering the wrath of RAGE: an emerging therapeutic strategy against diabetic complications, neurodegeneration, and inflammation. Ann Med. 2009; 41(6):408–422. PMID: 19322705.
Article6. Bierhaus A, Stern DM, Nawroth PP. RAGE in inflammation: a new therapeutic target? Curr Opin Investig Drugs. 2006; 7(11):985–991.7. Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992; 267(21):14998–15004. PMID: 1378843.
Article8. Stern D, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev. 2002; 54(12):1615–1625. PMID: 12453678.
Article9. Lohwasser C, Neureiter D, Popov Y, Bauer M, Schuppan D. Role of the receptor for advanced glycation end products in hepatic fibrosis. World J Gastroenterol. 2009; 15(46):5789–5798. PMID: 19998499.10. Basta G, Navarra T, De Simone P, Del Turco S, Gastaldelli A, Filipponi F. What is the role of the receptor for advanced glycation end products-ligand axis in liver injury? Liver Transpl. 2011; 17(6):633–640. PMID: 21438128.
Article11. Ekong U, Zeng S, Dun H, Feirt N, Guo J, Ippagunta N, et al. Blockade of the receptor for advanced glycation end products attenuates acetaminophen-induced hepatotoxicity in mice. J Gastroenterol Hepatol. 2006; 21(4):682–688. PMID: 16677153.
Article12. Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001; 33(7):637–668. PMID: 11390274.
Article13. Gonçalves CA, Leite MC, Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem. 2008; 41(10-11):755–763. PMID: 18454941.
Article14. Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, et al. S100B's double life: intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009; 1793(6):1008–1022. PMID: 19110011.
Article15. Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009; 1793(6):993–1007. PMID: 19121341.
Article16. Pelinka LE, Toegel E, Mauritz W, Redl H. Serum S 100 B: a marker of brain damage in traumatic brain injury with and without multiple trauma. Shock. 2003; 19(3):195–200. PMID: 12630517.
Article17. Raabe A, Kopetsch O, Woszczyk A, Lang J, Gerlach R, Zimmermann M, et al. Serum S-100B protein as a molecular marker in severe traumatic brain injury. Restor Neurol Neurosci. 2003; 21(3-4):159–169. PMID: 14530578.18. Steiner J, Bernstein HG, Bogerts B, Gos T, Richter-Landsberg C, Wunderlich MT, et al. S100B is expressed in, and released from, OLN-93 oligodendrocytes: Influence of serum and glucose deprivation. Neuroscience. 2008; 154(2):496–503. PMID: 18472341.
Article19. Netto CB, Conte S, Leite MC, Pires C, Martins TL, Vidal P, et al. Serum S100B protein is increased in fasting rats. Arch Med Res. 2006; 37(5):683–686. PMID: 16740441.
Article20. Pelinka LE, Harada N, Szalay L, Jafarmadar M, Redl H, Bahrami S. Release of S100B differs during ischemia and reperfusion of the liver, the gut, and the kidney in rats. Shock. 2004; 21(1):72–76. PMID: 14676687.
Article21. Starkel P, Leclercq IA. Animal models for the study of hepatic fibrosis. Best Pract Res Clin Gastroenterol. 2011; 25(2):319–333. PMID: 21497748.
Article22. Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, et al. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000; 41(6):882–893. PMID: 10828080.
Article23. Van Eldik LJ, Staecker JL, Winningham-Major F. Synthesis and expression of a gene coding for the calcium-modulated protein S100 beta and designed for cassette-based, site-directed mutagenesis. J Biol Chem. 1988; 263(16):7830–7837. PMID: 3372506.
Article24. Mely Y, Gérard D. Large-scale, one-step purification of oxidized and reduced forms of bovine brain S100b protein by HPLC. J Neurochem. 1988; 50(3):739–744. PMID: 3339349.
Article25. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979; 11(4):447–455. PMID: 91593.
Article26. Kim SE, Park JW, Kim MJ, Jang B, Jeon YC, Kim HJ, et al. Accumulation of citrullinated glial fibrillary acidic protein in a mouse model of bile duct ligation-induced hepatic fibrosis. PLoS One. 2018; 13(8):e0201744. PMID: 30071078.
Article27. Fehrenbach H, Weiskirchen R, Kasper M, Gressner AM. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology. 2001; 34(5):943–952. PMID: 11679965.
Article28. Cataldegirmen G, Zeng S, Feirt N, Ippagunta N, Dun H, Qu W, et al. RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB. J Exp Med. 2005; 201(3):473–484. PMID: 15699076.29. Yamagishi S, Matsui T. Role of receptor for advanced glycation end products (RAGE) in liver disease. Eur J Med Res. 2015; 20(1):15. PMID: 25888859.
Article30. Roskams T, van den Oord JJ, De Vos R, Desmet VJ. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol. 1990; 137(5):1019–1025. PMID: 1700614.31. Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol Res Pract. 1995; 191(6):513–524. PMID: 7479372.32. Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007; 132(1):415–431. PMID: 17241889.
Article33. Franchitto A, Onori P, Renzi A, Carpino G, Mancinelli R, Alvaro D, et al. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann Transl Med. 2013; 1(3):27. PMID: 25332971.34. Jensen K, Marzioni M, Munshi K, Afroze S, Alpini G, Glaser S. Autocrine regulation of biliary pathology by activated cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2012; 302(5):G473–83. PMID: 22194419.
Article35. Marzioni M, Francis H, Benedetti A, Ueno Y, Fava G, Venter J, et al. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tauroursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. Am J Pathol. 2006; 168(2):398–409. PMID: 16436655.
Article36. Heizmann CW, Fritz G, Schäfer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002; 7:d1356–68. PMID: 11991838.
Article37. Xia P, Deng Q, Gao J, Yu X, Zhang Y, Li J, et al. Therapeutic effects of antigen affinity-purified polyclonal anti-receptor of advanced glycation end-product (RAGE) antibodies on cholestasis-induced liver injury in rats. Eur J Pharmacol. 2016; 779:102–110. PMID: 26970185.
Article38. Iwamoto K, Kanno K, Hyogo H, Yamagishi S, Takeuchi M, Tazuma S, et al. Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J Gastroenterol. 2008; 43(4):298–304. PMID: 18458846.
Article39. Demetris AJ, Sever C, Kakizoe S, Oguma S, Starzl TE, Jaffe R. S100 protein positive dendritic cells in primary biliary cirrhosis and other chronic inflammatory liver diseases. Relevance to pathogenesis? Am J Pathol. 1989; 134(4):741–747. PMID: 2705505.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Liver Fibrosis by Bile Duct Sclerosis with Ethanol: a New Experimental Model for Liver Fibrosis
- Immunohistochemical Expression of Bcl-2 and Bax after Bile Duct Ligation in the Rat
- Temporal Morphologic Changes in the Mouse Liver after Common Bile Duct Ligation
- Experimental Animal Models of Hepatic Fibrosis
- A Technique for Bile Duct-Duodenal Anastomosis at the Consecutive Rat Liver Transplantation