Korean J Transplant.
2021 Mar;35(1):33-40. 10.4285/kjt.20.0052.
Clinical significance of de novo donor-specific antibody in kidney transplant recipients with chronic antibody-mediated rejection
- Affiliations
-
- 1Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea
- 2Keimyung University Kidney Institute, Daegu, Korea
- KMID: 2514406
- DOI: http://doi.org/10.4285/kjt.20.0052
Abstract
- Background
Chronic antibody-mediated rejection (CABMR) is an important cause of late graft loss. De novo donor-specific antibody (dnDSA) is an important prognostic factor for long-term allograft outcomes. However, the prognosis of CABMR based on the presence of dnDSA is uncertain.
Methods
We retrospectively analyzed 35 kidney transplant recipients with CABMR between 2010 and 2018. Fourteen recipients had no detectable DSA, and 21 recipients had detectable DSA. We investigated the pathologic findings at diagnosis of CABMR, allograft function 12 months later, related factors for allograft failure, and allograft survival rate based on the presence of dnDSA.
Results
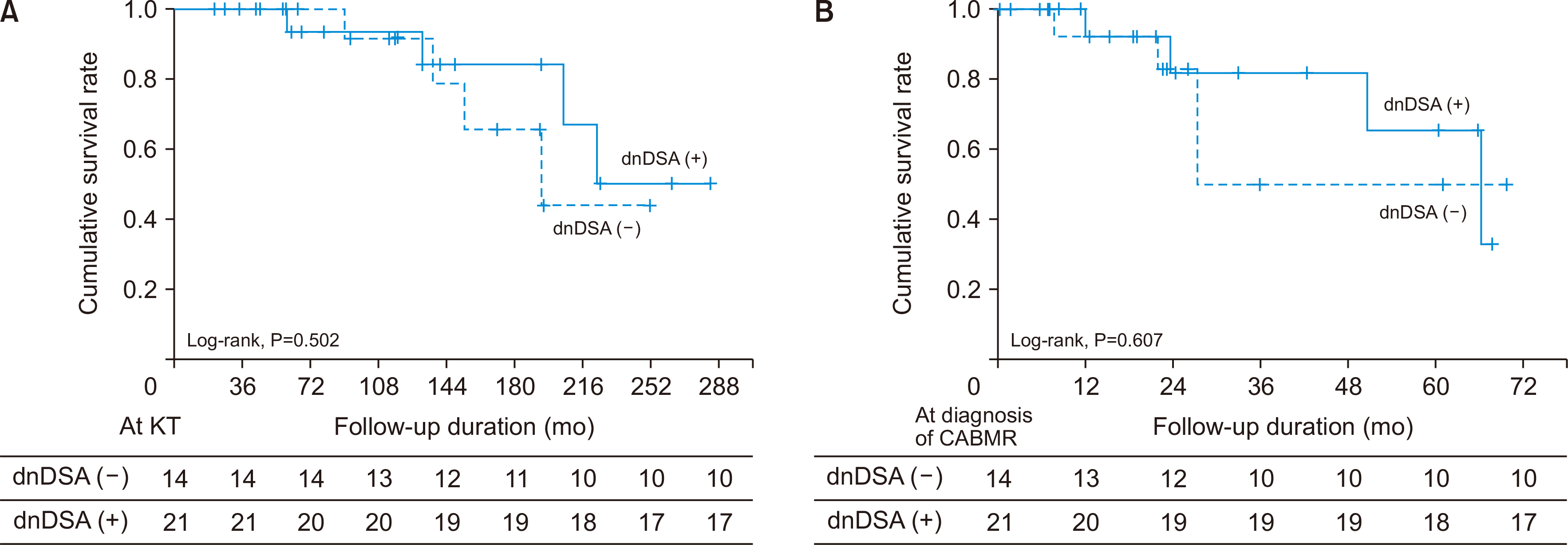
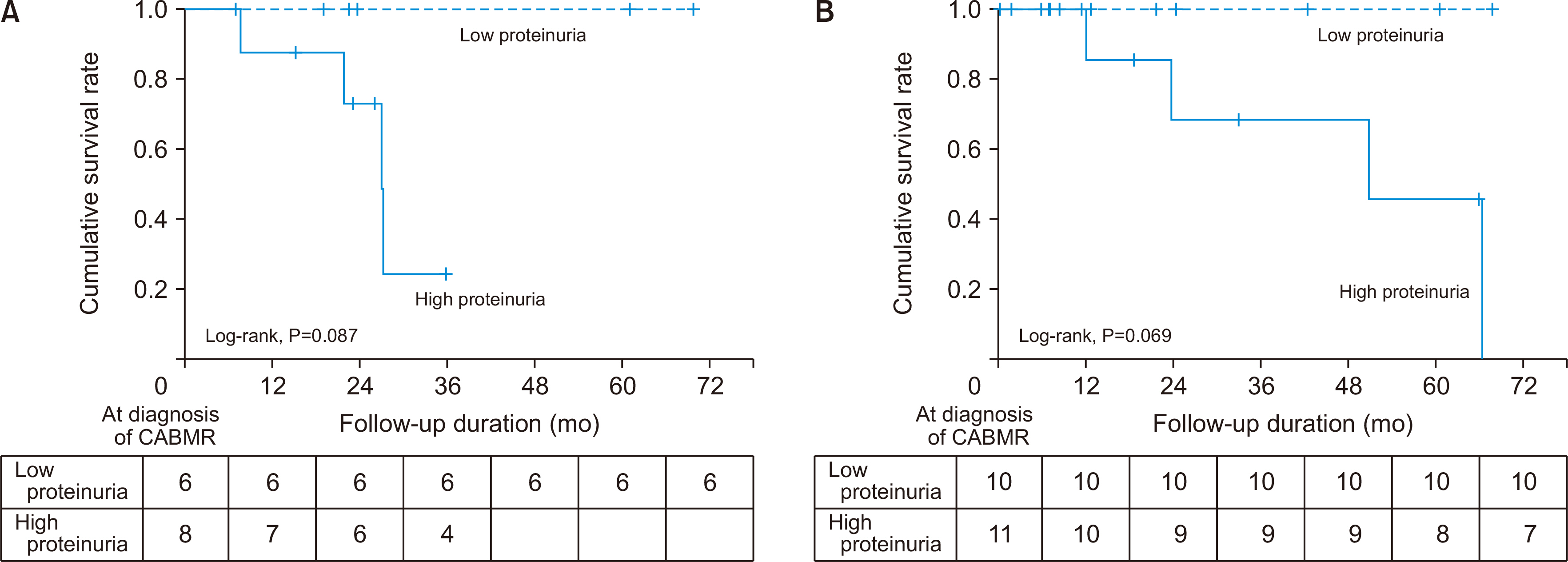
The pathologic findings showed that acute and chronic changes were more severe in the dnDSA (+) group than in the dnDSA (–) group. There was no significant differ-ence in the allograft function 12 months after the diagnosis of CABMR and in the amount of proteinuria at diagnosis between the two groups. However, the death-censored graft survival rate was lower in the high-proteinuria group than in the low-proteinuria group in both groups. The treatment rate of recipients was higher in the dnDSA (+) group than in the dnDSA (–) group; however, there was no significant difference in the death-censored graft survival rate between the two groups.
Conclusions
Although the effect of dnDSA on the prognosis of CABMR is not clear, it would be important not to neglect treatment for CABMR with risk factors for allograft failure even without dnDSA. Continuous and rigorous surveillance of DSA and allograft function is needed in patients with CABMR.
Figure
Cited by 1 articles
-
Impact of Low-Level Donor-Specific Anti-HLA Antibody on Posttransplant Clinical Outcomes in Kidney Transplant Recipients
Haeun Lee, Hanbi Lee, Sang Hun Eum, Eun Jeong Ko, Ji-Won Min, Eun-Jee Oh, Chul Woo Yang, Byung Ha Chung
Ann Lab Med. 2023;43(4):364-374. doi: 10.3343/alm.2023.43.4.364.
Reference
-
1. Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al. 2018; The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 18:293–307. DOI: 10.1111/ajt.14625. PMID: 29243394. PMCID: PMC5817248.
Article2. Singh N, Pirsch J, Samaniego M. 2009; Antibody-mediated rejection: treatment alternatives and outcomes. Transplant Rev (Orlando). 23:34–46. DOI: 10.1016/j.trre.2008.08.004. PMID: 19027615.
Article3. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. 2007; Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 53:766–72. DOI: 10.1373/clinchem.2006.077180. PMID: 17332152.
Article4. Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, et al. 2018; A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 102:1795–814. DOI: 10.1097/TP.0000000000002366. PMID: 30028786. PMCID: PMC7597974.
Article5. Solar-Cafaggi D, Marino L, Uribe-Uribe N, Morales-Buenrostro LE. 2018; Antibody-mediated rejection in the Banff classifications of 2007 and 2017: a comparison of renal graft loss prediction capability. Transpl Immunol. 51:40–4. DOI: 10.1016/j.trim.2018.08.008. PMID: 30170180.
Article6. Wan SS, Chadban SJ, Watson N, Wyburn K. 2020; Development and outcomes of de novo donor-specific antibodies in low, moderate, and high immunological risk kidney transplant recipients. Am J Transplant. 20:1351–64. DOI: 10.1111/ajt.15754. PMID: 31867849.
Article7. Haas M. 2016; The Revised (2013) Banff Classification for Antibody-Mediated Rejection of Renal Allografts: update, difficulties, and future considerations. Am J Transplant. 16:1352–7. DOI: 10.1111/ajt.13661. PMID: 26696524.
Article8. Ban TH, Yu JH, Chung BH, Choi BS, Park CW, Kim YS, et al. 2017; Clinical outcome of rituximab and intravenous immunoglobulin combination therapy in kidney transplant recipients with chronic active antibody-mediated rejection. Ann Transplant. 22:468–74. DOI: 10.12659/AOT.903499. PMID: 28775248.
Article9. Hassan R, Gheith O. 2014; Chronic antibody-mediated rejection: review of literature. Iran J Kidney Dis. 8:93–103. PMID: 24685730.10. Moreso F, Crespo M, Ruiz JC, Torres A, Gutierrez-Dalmau A, Osuna A, et al. 2018; Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: a multicenter, prospective, randomized, double-blind clinical trial. Am J Transplant. 18:927–35. DOI: 10.1111/ajt.14520. PMID: 28949089.
Article11. Gang S, Han A, Min S, Ha J, Yang J. 2019; Successful treatment of early acute antibody-mediated rejection in an human leukocyte antigen-incompatible and ABO-incompatible living-donor kidney transplant patient. Korean J Transplant. 33:153–8. DOI: 10.4285/jkstn.2019.33.4.153.
Article12. Zhang R. 2018; Donor-specific antibodies in kidney transplant recipients. Clin J Am Soc Nephrol. 13:182–92. DOI: 10.2215/CJN.00700117. PMID: 28446536. PMCID: PMC5753302.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathologic Updates on Antibody Mediated Rejection in Renal Transplantation
- Comparison of clinical and pathological features of rejection in ABO-incompatible and ABO-compatible kidney transplantation
- Evaluating the effect of Anti-HLA-DR51/52/53 donor-specific antibodies on antibody-mediated rejection in kidney transplantation recipients
- New treatment for antibody-mediated rejection: interleukin-6 inhibitors
- Investigation of non-human leukocyte antigen antibodies and epitope mismatch in kidney transplant recipients with chronic antibody-mediated rejection