Korean J Transplant.
2021 Mar;35(1):8-14. 10.4285/kjt.20.0043.
Early use of everolimus improved renal function after adult deceased donor liver transplantation
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2514403
- DOI: http://doi.org/10.4285/kjt.20.0043
Abstract
- Background
Tacrolimus (TAC) is a main therapy for liver transplantation (LT) patients, but it has side effects such as chronic nephrotoxicity that progressively aggravate renal function. The purpose of this study was to retrospectively compare the renal function between a TAC group and a combination of everolimus and reduced TAC (EVR-TAC) group after deceased donor liver transplantation (DDLT).
Methods
The study comprised 131 patients who underwent DDLT between January 2013 and April 2018 at our institution. They received TAC or EVR-TAC after DDLT. Everolimus (EVR) was introduced between 1 and 6 months after DDLT.
Results
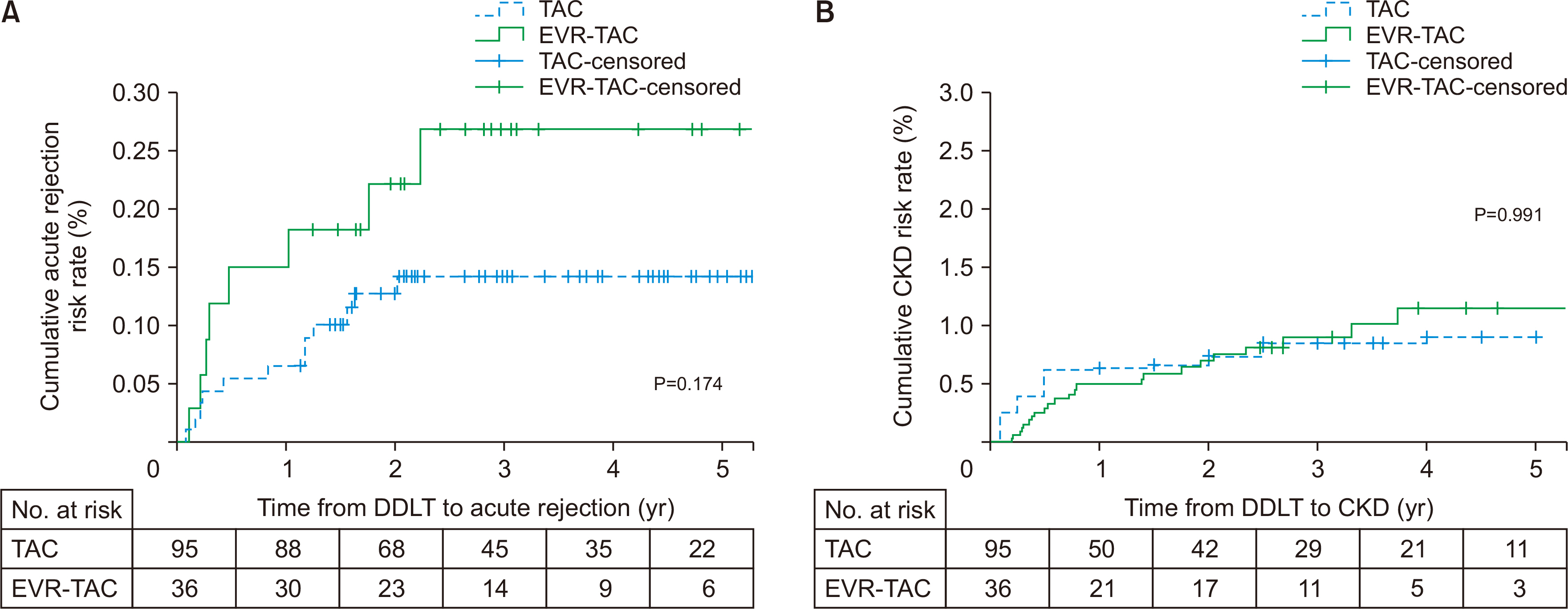
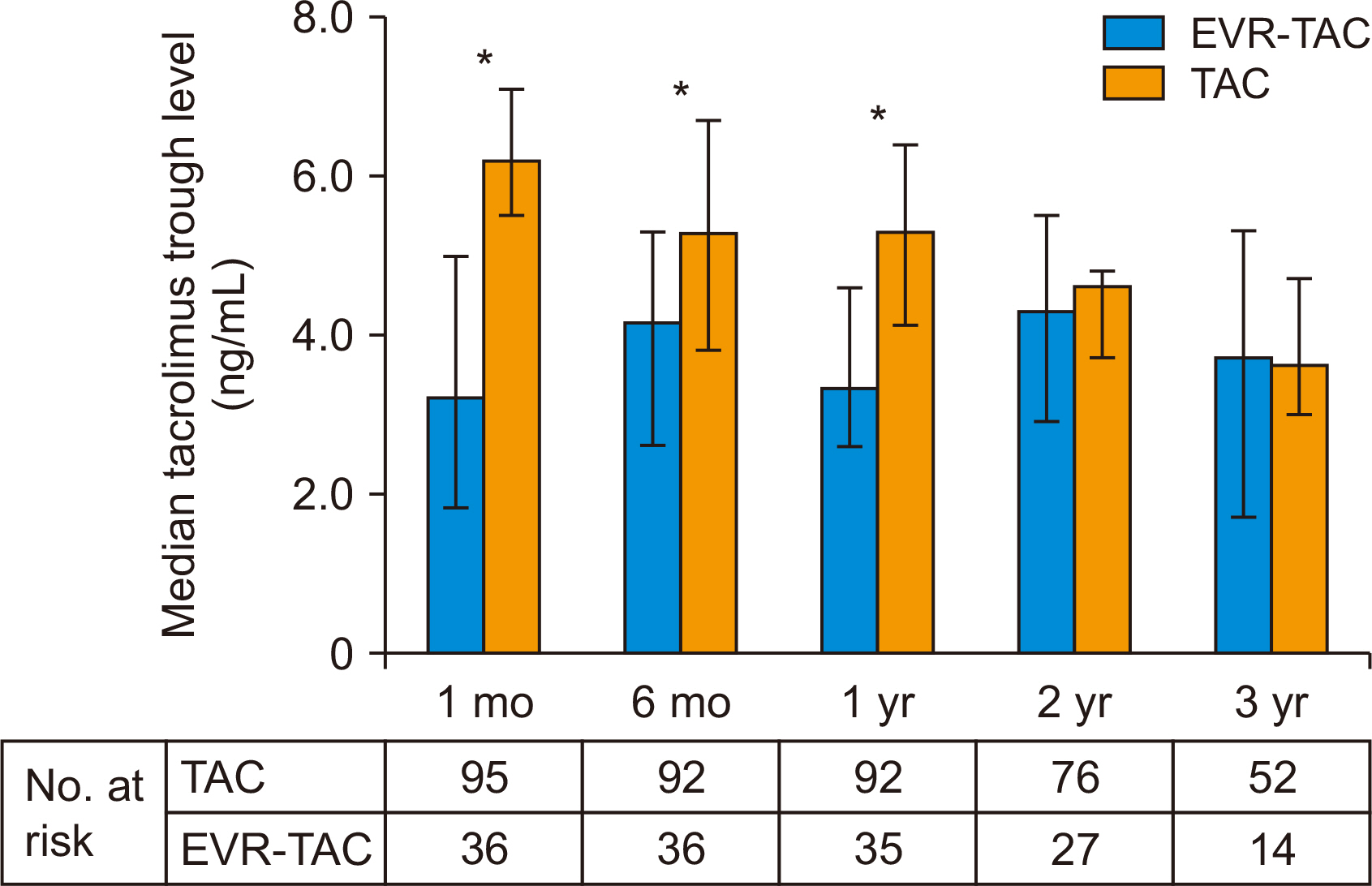
Thirty-six of 131 patients (27.5%) received EVR-TAC. The incidence of chronic kidney disease (CKD; estimated glomerular filtration rate, <60 mL/1.73 m 2 ) in the EVR- TAC group was higher than in the TAC group (25% vs. 8.4%; P=0.019). Increasing serum creatinine (n=23, 63.9%) was the most common cause for adding EVR to treatment of the posttransplant patients. There were no statistical differences in acute rejection and CKD between the two groups. The TAC trough level was significantly lower in the EVRTAC group than in the TAC group, and the renal function of the EVR-TAC group was worse than that of the TAC group until 1 year after DDLT. However, the renal function of the EVRTAC group improved and became similar to that of TAC group at 3 years posttransplant.
Conclusions
The present study suggests that EVR should be introduced as soon as possible after DDLT to reduce exposure to high doses of TAC to improve the renal function.
Figure
Reference
-
1. Brunet M, van Gelder T, Åsberg A, Haufroid V, Hesselink DA, Langman L, et al. 2019; Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 41:261–307. DOI: 10.1097/FTD.0000000000000640. PMID: 31045868.
Article2. Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. 2003; Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 349:931–40. DOI: 10.1056/NEJMoa021744. PMID: 12954741.
Article3. Saliba F, Fischer L, de Simone P, Bernhardt P, Bader G, Fung J. 2018; Association between renal dysfunction and major adverse cardiac events after liver transplantation: evidence from an international randomized trial of everolimus-based immunosuppression. Ann Transplant. 23:751–7. DOI: 10.12659/AOT.911030. PMID: 30361470. PMCID: PMC6248043.
Article4. Saliba F, De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, et al. 2013; Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 13:1734–45. DOI: 10.1111/ajt.12280. PMID: 23714399.
Article5. Fischer L, Saliba F, Kaiser GM, De Carlis L, Metselaar HJ, De Simone P, et al. 2015; Three-year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: follow-up results from a randomized, multicenter study. Transplantation. 99:1455–62. DOI: 10.1097/TP.0000000000000555. PMID: 26151607.6. Saliba F. 2017; Everolimus-based immunosuppression in liver transplant recipients. Am J Transplant. 17:2489. DOI: 10.1111/ajt.14388. PMID: 28654719.
Article7. Guan TW, Lin YJ, Ou MY, Chen KB. 2019; Efficacy and safety of everolimus treatment on liver transplant recipients: a meta-analysis. Eur J Clin Invest. 49:e13179. DOI: 10.1111/eci.13179. PMID: 31610022.
Article8. Saliba F, Duvoux C, Gugenheim J, Kamar N, Dharancy S, Salamé E, et al. 2017; Efficacy and safety of everolimus and mycophenolic acid with early tacrolimus withdrawal after liver transplantation: a multicenter randomized trial. Am J Transplant. 17:1843–52. DOI: 10.1111/ajt.14212. PMID: 28133906.
Article9. Kang SH, Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, et al. 2019; Cross-sectional analysis of immunosuppressive regimens focused on everolimus after liver transplantation in a Korean high-volume transplantation center. Korean J Transplant. 33:98–105. DOI: 10.4285/jkstn.2019.33.4.98.
Article10. Kim JM, Lee KW, Song GW, Jung BH, Lee HW, Yi NJ, et al. 2016; Immunosuppression status of liver transplant recipients with hepatitis C affects biopsy-proven acute rejection. Clin Mol Hepatol. 22:366–71. DOI: 10.3350/cmh.2016.0022. PMID: 27729628. PMCID: PMC5066384.
Article11. Demetris AJ, Adeyi O, Bellamy CO, Clouston A, Charlotte F, et al. Banff Working Group. 2006; Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 44:489–501. DOI: 10.1002/hep.21280. PMID: 16871565.
Article12. Trinh E, Alam A, Tchervenkov J, Cantarovich M. 2017; Impact of acute kidney injury following liver transplantation on long-term outcomes. Clin Transplant. 31:e12863. DOI: 10.1111/ctr.12863. PMID: 27801506.
Article13. Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. 2014; Chronic kidney disease and associated mortality after liver transplantation: a time-dependent analysis using measured glomerular filtration rate. J Hepatol. 61:286–92. DOI: 10.1016/j.jhep.2014.03.034. PMID: 24713190. PMCID: PMC4160310.14. De Simone P, Saliba F, Dong G, Escrig C, Fischer L. 2016; Do patient characteristics influence efficacy and renal outcomes in liver transplant patients receiving everolimus? Clin Transplant. 30:279–88. DOI: 10.1111/ctr.12687. PMID: 26717035.
Article15. Rodríguez-Perálvarez M, Germani G, Darius T, Lerut J, Tsochatzis E, Burroughs AK. 2012; Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 12:2797–814. DOI: 10.1111/j.1600-6143.2012.04140.x. PMID: 22703529.
Article16. Rodríguez-Perálvarez M, Germani G, Papastergiou V, Tsochatzis E, Thalassinos E, Luong TV, et al. 2013; Early tacrolimus exposure after liver transplantation: relationship with moderate/severe acute rejection and long-term outcome. J Hepatol. 58:262–70. DOI: 10.1016/j.jhep.2012.09.019. PMID: 23023010.
Article17. Lerut J, Mathys J, Verbaandert C, Talpe S, Ciccarelli O, Lemaire J, et al. 2008; Tacrolimus monotherapy in liver transplantation: one-year results of a prospective, randomized, double-blind, placebo-controlled study. Ann Surg. 248:956–67. DOI: 10.1097/SLA.0b013e31819009c9. PMID: 19092340.18. Tang CY, Shen A, Wei XF, Li QD, Liu R, Deng HJ, et al. 2015; Everolimus in de novo liver transplant recipients: a systematic review. Hepatobiliary Pancreat Dis Int. 14:461–9. DOI: 10.1016/S1499-3872(15)60419-2. PMID: 26459721.
Article19. Yee ML, Tan HH. 2017; Use of everolimus in liver transplantation. World J Hepatol. 9:990–1000. DOI: 10.4254/wjh.v9.i23.990. PMID: 28878864. PMCID: PMC5569278.
Article20. De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, et al. 2012; Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 12:3008–20. DOI: 10.1111/j.1600-6143.2012.04212.x. PMID: 22882750. PMCID: PMC3533764.21. De Simone P, Metselaar HJ, Fischer L, Dumortier J, Boudjema K, Hardwigsen J, et al. 2009; Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: a prospective, randomized, multicenter trial. Liver Transpl. 15:1262–9. DOI: 10.1002/lt.21827. PMID: 19790150.
Article22. Saliba F, Duvoux C, Dharancy S, Dumortier J, Calmus Y, Gugenheim J, et al. 2019; Early switch from tacrolimus to everolimus after liver transplantation: outcomes at 2 years. Liver Transpl. 25:1822–32. DOI: 10.1002/lt.25664. PMID: 31631501. PMCID: PMC7383505.
Article23. Saliba F, Dharancy S, Lorho R, Conti F, Radenne S, Neau-Cransac M, et al. 2011; Conversion to everolimus in maintenance liver transplant patients: a multicenter, retrospective analysis. Liver Transpl. 17:905–13. DOI: 10.1002/lt.22292. PMID: 21384525.
Article24. Holdaas H, Rostaing L, Serón D, Cole E, Chapman J, Fellstrøm B, et al. 2011; Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation. 92:410–8. DOI: 10.1097/TP.0b013e318224c12d. PMID: 21697773.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Liver retransplantation for adult recipients
- Experience of split liver transplantation from deceased marginal donor: eight adult recipients from four deceased donors
- Current Status of Liver Transplantation in Korea
- Split liver transplantation for two adult recipients: A collective review of Korean experience
- Left at right heterotopic implantation of left liver graft in adult-to-adult living donor liver transplantation: the technical concern for decision-making