Clin Endosc.
2021 Mar;54(2):161-181. 10.5946/ce.2021.069.
Clinical and Technical Guideline for Endoscopic Ultrasound (EUS)-Guided Tissue Acquisition of Pancreatic Solid Tumor: Korean Society of Gastrointestinal Endoscopy (KSGE)
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Gastroenterology, Department of Internal Medicine, Hallym University College of Medicine, Hwaseong, Korea
- 3Department of Internal Medicine, Research Institute of Clinical Medicine of Jeonbuk National University-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, Korea
- 4Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea
- 5Division of Gastroenterology, Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
- 6Division of Gastroenterology, Department of Internal Medicine, Jeju National University College of Medicine, Jeju, Korea
- 7Division of Gastroenterology, Department of Internal Medicine, Soonchunhyang University College of Medicine, Cheonan, Korea
- 8Division of Gastroenterology, Department of Internal Medicine, Chonnam National University College of Medicine, Gwangju, Korea
- 9Division of Gastroenterology, Department of Internal Medicine, Dongguk University College of Medicine, Goyang, Korea
- 10Division of Gastroenterology, Department of Internal Medicine, Ulsan University College of Medicine, Seoul, Korea
- 11Division of Gastroenterology, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- 12Division of Gastroenterology, Department of Internal Medicine, Inje University College of Medicine, Goyang, Korea
- 13Division of Gastroenterology, Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea
- 14Division of Gastroenterology, Department of Internal Medicine, Kyung Hee University College of Medicine, Seoul, Korea
- 15Division of Healthcare Technology Assessment Research, Office of Health Technology Assessment Research, National Evidence-based Healthcare Collaborating Agency, Seoul, Korea
- 16Division of Gastroenterology, Department of Internal Medicine, Catholic University of Daegu College of Medicine, Daegu, Korea
- 17Division of Gastroenterology, Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea
- 18Division of Gastroenterology, Department of Internal Medicine, Chosun University College of Medicine, Korea, Gwangju, Korea
- 19Division of Gastroenterology, Department of Internal Medicine, Cha University College of Medicine, Seongnam, Korea
- KMID: 2514171
- DOI: http://doi.org/10.5946/ce.2021.069
Abstract
- Endoscopic ultrasound (EUS)-guided tissue acquisition of pancreatic solid tumor requires a strict recommendation for its proper use in clinical practice because of its technical difficulty and invasiveness. The Korean Society of Gastrointestinal Endoscopy (KSGE) appointed a Task Force to draft clinical practice guidelines for EUS-guided tissue acquisition of pancreatic solid tumor. The strength of recommendation and the level of evidence for each statement were graded according to the Minds Handbook for Clinical Practice Guideline Development 2014. The committee, comprising a development panel of 16 endosonographers and an expert on guideline development methodology, developed 12 evidence-based recommendations in 8 categories intended to help physicians make evidence-based clinical judgments with regard to the diagnosis of pancreatic solid tumor. This clinical practice guideline discusses EUS-guided sampling in pancreatic solid tumor and makes recommendations on circumstances that warrant its use, technical issues related to maximizing the diagnostic yield (e.g., needle type, needle diameter, adequate number of needle passes, sample obtaining techniques, and methods of specimen processing), adverse events of EUS-guided tissue acquisition, and learning-related issues. This guideline was reviewed by external experts and suggests best practices recommended based on the evidence available at the time of preparation. This guideline may not be applicable for all clinical situations and should be interpreted in light of specific situations and the availability of resources. It will be revised as necessary to cover progress and changes in technology and evidence from clinical practice.
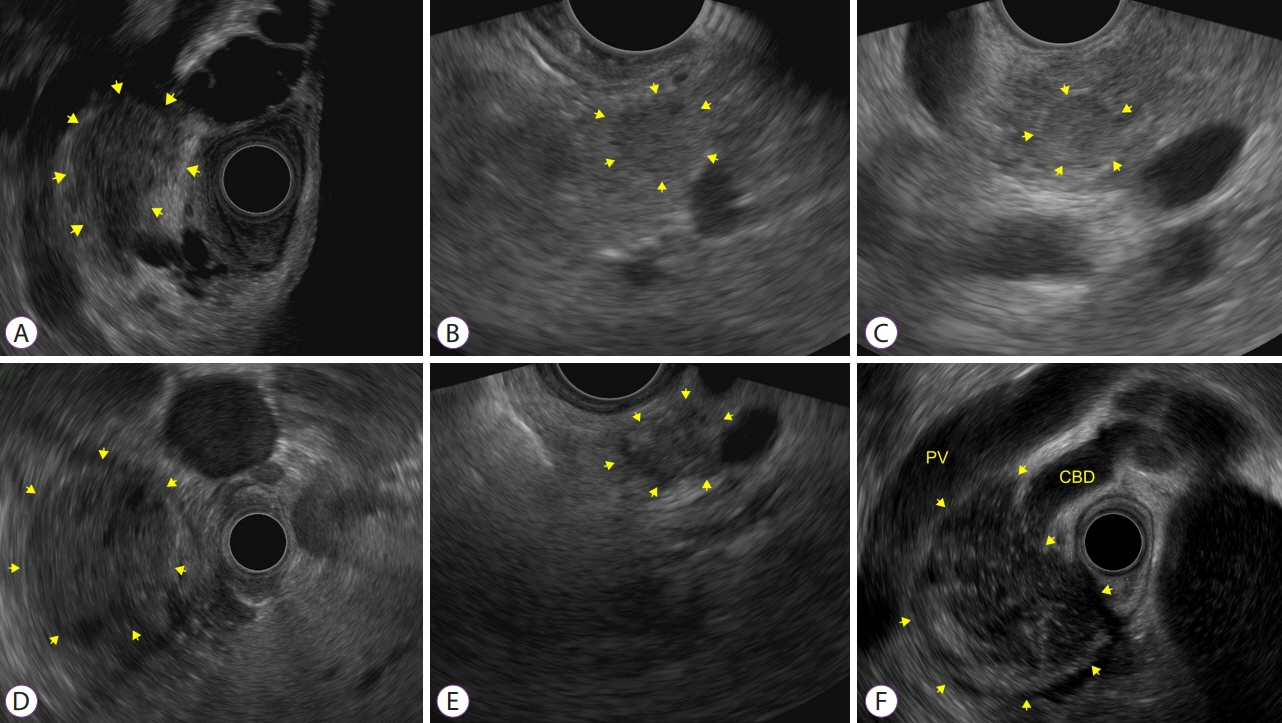
Figure
Reference
-
1. Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992; 38:172–173.
Article2. Eisen GM, Chutkan R, Goldstein JL, et al. Role of endoscopic ultrasonography. Gastrointest Endosc. 2000; 52:852–859.
Article3. Maluf-Filho F, Dotti CM, Halwan B, et al. An evidence-based consensus statement on the role and application of endosonography in clinical practice. Endoscopy. 2009; 41:979–987.
Article4. Kida M. Pancreatic masses. Gastrointest Endosc. 2009; 69:S102–S109.
Article5. Park SW, Chung MJ, Lee SH, et al. Prospective study for comparison of endoscopic ultrasound-guided tissue acquisition using 25- and 22-gauge core biopsy needles in solid pancreatic masses. PLoS One. 2016; 11:e0154401.
Article6. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019; 10:ED000142.
Article7. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898.
Article8. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–605.
Article9. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–536.
Article10. Yokoe M, Takada T, Mayumi T, et al. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci. 2015; 22:405–432.
Article11. Park SK, Ye BD, Kim KO, et al. Guidelines for video capsule endoscopy: emphasis on Crohn’s disease. Clin Endosc. 2015; 48:128–135.
Article12. Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011; 43:897–912.
Article13. Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013; 24:159–171.
Article14. Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012; 75:319–331.
Article15. Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: a meta-analysis and systematic review. Pancreas. 2013; 42:20–26.16. Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003; 58:690–695.
Article17. van Gulik TM, Reeders JW, Bosma A, et al. Incidence and clinical findings of benign, inflammatory disease in patients resected for presumed pancreatic head cancer. Gastrointest Endosc. 1997; 46:417–423.
Article18. Ang TL, Kwek AB, Seo DW, et al. A prospective randomized study of the difference in diagnostic yield between endoscopic ultrasound-guided fine-needle aspiration (EUSFNA) needles with and without a side port in pancreatic masses. Endosc Int Open. 2015; 3:E329–E333.
Article19. Facciorusso A, Wani S, Triantafyllou K, et al. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: a network meta-analysis. Gastrointest Endosc. 2019; 90:893–903.e7.
Article20. Kamata K, Kitano M, Yasukawa S, et al. Histologic diagnosis of pancreatic masses using 25-gauge endoscopic ultrasound needles with and without a core trap: a multicenter randomized trial. Endoscopy. 2016; 48:632–638.
Article21. Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000; 51:184–190.
Article22. LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004; 59:475–481.
Article23. Bang JY, Hebert-Magee S, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012; 76:321–327.
Article24. Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013; 45:445–450.
Article25. Möller K, Papanikolaou IS, Toermer T, et al. EUS-guided FNA of solid pancreatic masses: high yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009; 70:60–69.
Article26. Pitman MB, Centeno BA, Ali SZ, et al. Standardized terminology and nomenclature for pancreatobiliary cytology: the Papanicolaou Society of Cytopathology Guidelines. Cytojournal. 2014; 11:3.
Article27. Kliment M, Urban O, Cegan M, et al. Endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: the utility and impact on management of patients. Scand J Gastroenterol. 2010; 45:1372–1379.
Article28. Horwhat JD, Paulson EK, McGrath K, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006; 63:966–975.
Article29. Eloubeidi MA, Varadarajulu S, Desai S, Wilcox CM. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol. 2008; 23:567–570.
Article30. Layfield LJ, Schmidt RL, Hirschowitz SL, Olson MT, Ali SZ, Dodd LL. Significance of the diagnostic categories “atypical” and “suspicious for malignancy” in the cytologic diagnosis of solid pancreatic masses. Diagn Cytopathol. 2014; 42:292–296.
Article31. Fuccio L, Hassan C, Laterza L, et al. The role of K-ras gene mutation analysis in EUS-guided FNA cytology specimens for the differential diagnosis of pancreatic solid masses: a meta-analysis of prospective studies. Gastrointest Endosc. 2013; 78:596–608.32. Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011; 106:1705–1710.
Article33. Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015; 110:1429–1439.
Article34. Ganc RL, Carbonari AP, Colaiacovo R, et al. Rapid on-site cytopathological examination (ROSE) performed by endosonagraphers and its improvement in the diagnosis of pancreatic solid lesions. Acta Cir Bras. 2015; 30:503–508.
Article35. Cermak TS, Wang B, DeBrito P, Carroll J, Haddad N, Sidawy MK. Does on-site adequacy evaluation reduce the nondiagnostic rate in endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions? Cancer Cytopathol. 2012; 120:319–325.
Article36. Kong F, Zhu J, Kong X, et al. Rapid on-site evaluation does not improve endoscopic ultrasound-guided fine needle aspiration adequacy in pancreatic masses: a meta-analysis and systematic review. PLoS One. 2016; 11:e0163056.
Article37. Matynia AP, Schmidt RL, Barraza G, Layfield LJ, Siddiqui AA, Adler DG. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014; 29:697–705.
Article38. Kim JH, Park SW, Kim MK, et al. Meta-analysis for cyto-pathological outcomes in endoscopic ultrasonography-guided fine-needle aspiration with and without the stylet. Dig Dis Sci. 2016; 61:2175–2184.
Article39. Sahai AV, Paquin SC, Gariépy G. A prospective comparison of endoscopic ultrasound-guided fine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2010; 42:900–903.
Article40. Wani S, Early D, Kunkel J, et al. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: a prospective, single blind, randomized, controlled trial. Gastrointest Endosc. 2012; 76:328–335.
Article41. Puri R, Vilmann P, Saftoiu A, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009; 44:499–504.
Article42. Lee JK, Choi JH, Lee KH, et al. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013; 77:745–751.
Article43. Kudo T, Kawakami H, Hayashi T, et al. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014; 80:1030–1037.e1.
Article44. Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014; 59:1578–1585.
Article45. Matsubayashi H, Matsui T, Yabuuchi Y, et al. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol. 2016; 22:628–640.
Article46. Lee KY, Cho HD, Hwangbo Y, et al. Efficacy of 3 fine-needle biopsy techniques for suspected pancreatic malignancies in the absence of an on-site cytopathologist. Gastrointest Endosc. 2019; 89:825–831.e1.
Article47. Bor R, Vasas B, Fábián A, et al. Prospective comparison of slow-pull and standard suction techniques of endoscopic ultrasound-guided fine needle aspiration in the diagnosis of solid pancreatic cancer. BMC Gastroenterol. 2019; 19:6.
Article48. Park SW, Lee SS, Song TJ, et al. The diagnostic performance of novel torque technique for EUS-guided tissue acquisition in solid pancreatic lesions: A prospective randomized controlled trial. J Gastroenterol Hepatol . 2020; 35:508–515.49. Matsubayashi H, Sasaki K, Ono S, et al. Pathological and molecular aspects to improve endoscopic ultrasonography-guided fine-needle aspiration from solid pancreatic lesions. Pancreas. 2018; 47:163–172.
Article50. Kocjan G, Chandra A, Cross P, et al. BSCC Code of Practice--fine needle aspiration cytology. Cytopathology. 2009; 20:283–296.51. Thierry R, Marie-Christine R, Marièle M, Richard B, Anca M. Modified technique of toluidine blue staining in rapid on-site evaluation. Diagn Cytopathol. 2012; 40:847–848.
Article52. Lee YN, Moon JH, Kim HK, et al. A triple approach for diagnostic assessment of endoscopic ultrasound-guided fine needle aspiration in pancreatic solid masses and lymph nodes. Dig Dis Sci. 2014; 59:2286–2293.
Article53. Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008; 111:167–177.54. Siddiqui MT, Gokaslan ST, Saboorian MH, Ashfaq R. Split sample comparison of ThinPrep and conventional smears in endoscopic retrograde cholangiopancreatography-guided pancreatic fine-needle aspirations. Diagn Cytopathol. 2005; 32:70–75.
Article55. Qin SY, Zhou Y, Li P, Jiang H-X. Diagnostic efficacy of cell block immunohistochemistry, smear cytology, and liquid-based cytology in endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions: a single-institution experience. PLoS One. 2014; 9:e108762.
Article56. LeBlanc JK, Emerson RE, Dewitt J, et al. A prospective study comparing rapid assessment of smears and ThinPrep for endoscopic ultrasound-guided fine-needle aspirates. Endoscopy. 2010; 42:389–394.
Article57. Lee JK, Choi ER, Jang TH, et al. A prospective comparison of liquid-based cytology and traditional smear cytology in pancreatic endoscopic ultrasound-guided fine needle aspiration. Acta Cytol. 2011; 55:401–407.
Article58. Lee KJ, Kang YS, Cho MY, Kim JW. Comparison of cytologic preparation methods in endoscopic ultrasound-guided fine needle aspiration for diagnosis of pancreatic adenocarcinoma. Pancreatology. 2016; 16:824–828.
Article59. Ieni A, Barresi V, Todaro P, Caruso RA, Tuccari G. Cell-block procedure in endoscopic ultrasound-guided-fine-needle-aspiration of gastrointestinal solid neoplastic lesions. World J Gastrointest Endosc. 2015; 7:1014–1022.
Article60. Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010; 45:868–875.
Article61. Facciorusso A, Wani S, Triantafyllou K, et al. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: a network meta-analysis. Gastrointest Endosc. 2019; 90:893–903.e7.
Article62. Rusch M, Nakitandwe J, Shurtleff S, et al. Clinical cancer genomic profiling by three-platform sequencing of whole genome, whole exome and transcriptome. Nat Commun. 2018; 9:3962.
Article63. Gao L, Antic T, Hyjek E, et al. Immunohistochemical analysis of E-cadherin and zeste homolog 2 expression in endoscopic ultrasound-guided fine-needle aspiration of pancreatic adenocarcinoma. Cancer Cytopathol. 2013; 121:644–652.
Article64. Dim DC, Jiang F, Qiu Q, et al. The usefulness of S100P, mesothelin, fascin, prostate stem cell antigen, and 14-3-3 sigma in diagnosing pancreatic adenocarcinoma in cytological specimens obtained by endoscopic ultrasound guided fine-needle aspiration. Diagn Cytopathol. 2014; 42:193–199.
Article65. Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014; 46:32–38.
Article66. O’Brien MJ, Takahashi M, Brugal G, et al. Digital imagery/telecytology. International Academy of Cytology Task Force summary. Diagnostic Cytology Towards the 21st Century: An International Expert Conference and Tutorial. Acta Cytol. 1998; 42:148–164.67. Kim B, Chhieng DC, Crowe DR, et al. Dynamic telecytopathology of on site rapid cytology diagnoses for pancreatic carcinoma. Cytojournal. 2006; 3:27.
Article68. Nasuti JF, Gupta PK, Baloch ZW. Diagnostic value and cost-effectiveness of on-site evaluation of fine-needle aspiration specimens: review of 5,688 cases. Diagn Cytopathol. 2002; 27:1–4.
Article69. Alsharif M, Carlo-Demovich J, Massey C, et al. Telecytopathology for immediate evaluation of fine-needle aspiration specimens. Cancer Cytopathol. 2010; 118:119–126.
Article70. Marotti JD, Johncox V, Ng D, Gonzalez JL, Padmanabhan V. Implementation of telecytology for immediate assessment of endoscopic ultrasound-guided fine-needle aspirations compared to conventional on-site evaluation: analysis of 240 consecutive cases. Acta Cytol. 2012; 56:548–553.
Article71. Brosens LA, Hackeng WM, Offerhaus GJ, Hruban RH, Wood LD. Pancreatic adenocarcinoma pathology: changing “landscape”. J Gastrointest Oncol. 2015; 6:358–374.72. Matsubayashi H, Watanabe H, Ajioka Y, et al. Different amounts of K-ras mutant epithelial cells in pancreatic carcinoma and mass-forming pancreatitis. Pancreas. 2000; 21:77–85.
Article73. Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016; 2:16022.
Article74. Levy MJ, Oberg TN, Campion MB, et al. Comparison of methods to detect neoplasia in patients undergoing endoscopic ultrasound-guided fine-needle aspiration. Gastroenterology. 2012; 142:1112–1121.e2.
Article75. Ribeiro A, Peng J, Casas C, Fan Y-S. Endoscopic ultrasound guided fine needle aspiration with fluorescence in situ hybridization analysis in 104 patients with pancreatic mass. J Gastroenterol Hepatol. 2014; 29:1654–1658.
Article76. Koorstra J-BM, Hustinx SR, Offerhaus GJA, Maitra A. Pancreatic carcinogenesis. Pancreatology. 2008; 8:110–125.
Article77. Jhala NC, Eltoum IA, Eloubeidi MA, et al. Providing on-site diagnosis of malignancy on endoscopic-ultrasound-guided fine-needle aspirates: should it be done? Ann Diagn Pathol. 2007; 11:176–181.
Article78. Oshima M, Okano K, Muraki S, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg. 2013; 258:336–346.
Article79. Lee J, Jang KT, Ki CS, et al. Impact of epidermal growth factor receptor (EGFR) kinase mutations, EGFR gene amplifications, and KRAS mutations on survival of pancreatic adenocarcinoma. Cancer. 2007; 109:1561–1569.80. Kobayashi M, Mizuno S, Murata Y, et al. Gemcitabine-based chemoradiotherapy followed by surgery for borderline resectable and locally unresectable pancreatic ductal adenocarcinoma: significance of the CA19-9 reduction rate and intratumoral human equilibrative nucleoside transporter 1 expression. Pancreas. 2014; 43:350–360.81. Fujita H, Ohuchida K, Mizumoto K, et al. High EGFR mRNA expression is a prognostic factor for reduced survival in pancreatic cancer after gemcitabine-based adjuvant chemotherapy. Int J Oncol. 2011; 38:629–641.
Article82. Ma G, Sun Y, Fu S. Evaluation of S100A4 mRNA in EUS-FNA specimens for the assessment of chemosensitivity to gemcitabine from patients with unresectable pancreatic cancer. Int J Clin Exp Pathol. 2015; 8:13284–13288.83. Preis M, Gardner TB, Gordon SR, et al. MicroRNA-10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011; 17:5812–5821.
Article84. Duconseil P, Gilabert M, Gayet O, et al. Transcriptomic analysis predicts survival and sensitivity to anticancer drugs of patients with a pancreatic adenocarcinoma. Am J Pathol. 2015; 185:1022–1032.
Article85. Wakatsuki T, Irisawa A, Imamura H, et al. Complete response of anaplastic pancreatic carcinoma to paclitaxel treatment selected by chemosensitivity testing. Int J Clin Oncol. 2010; 15:310–313.
Article86. Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011; 73:283–290.
Article87. Eloubeidi MA, Gress FG, Savides TJ, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004; 60:385–389.
Article88. Rodríguez-D’Jesús A, Fernández-Esparrach G, Marra-Lopez C, et al. Adverse events of EUS-guided FNA of pancreatic cystic and solid lesions by using the lexicon proposed in an ASGE workshop: a prospective and comparative study. Gastrointest Endosc. 2016; 83:780–784.89. Fernández-Esparrach G, Ginès A, García P, et al. Incidence and clinical significance of hyperamylasemia after endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of pancreatic lesions: a prospective and controlled study. Endoscopy. 2007; 39:720–724.
Article90. Gress FG, Barawi M, Kim D, Grendell JH. Preoperative localization of a neuroendocrine tumor of the pancreas with EUS-guided fine needle tattooing. Gastrointest Endosc. 2002; 55:594–597.
Article91. Katanuma A, Maguchi H, Yane K, et al. Factors predictive of adverse events associated with endoscopic ultrasound-guided fine needle aspiration of pancreatic solid lesions. Dig Dis Sci. 2013; 58:2093–2099.
Article92. Fujii LL, Levy MJ. Basic techniques in endoscopic ultrasound-guided fine needle aspiration for solid lesions: Adverse events and avoiding them. Endosc Ultrasound. 2014; 3:35–45.
Article93. Janssen J, König K, Knop-Hammad V, Johanns W, Greiner L. Frequency of bacteremia after linear EUS of the upper GI tract with and without FNA. Gastrointest Endosc. 2004; 59:339–344.
Article94. Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: a retrospective, comparative analysis. Gastrointest Endosc. 2011; 74:81–86.
Article95. ASGE Standards Of Practice Committee, Banerjee S, Shen B, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008; 67:791–798.96. Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012; 44:190–206.
Article97. Hamada T, Yasunaga H, Nakai Y, et al. Severe bleeding and perforation are rare complications of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: an analysis of 3,090 patients from 212 hospitals. Gut Liver. 2014; 8:215–258.
Article98. Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017. Endoscopy. 2017; 49:695–714.
Article99. Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Acute extraluminal hemorrhage associated with EUS-guided fine needle aspiration: frequency and clinical significance. Gastrointest Endosc. 2001; 53:221–225.
Article100. Varadarajulu S, Eloubeidi MA. Frequency and significance of acute intracystic hemorrhage during EUS-FNA of cystic lesions of the pancreas. Gastrointest Endosc. 2004; 60:631–635.
Article101. Veitch AM, Vanbiervliet G, Gershlick AH, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut. 2016; 65:374–389.
Article102. ASGE Standards of Practice Committee, Eloubeidi MA, Decker GA, et al. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc. 2016; 83:17–28.103. Minaga K, Kitano M, Enoki E, Kashida H, Kudo M. Needle-tract seeding on the proximal gastric wall after EUS-guided fine-needle aspiration of a pancreatic mass. Am J Gastroenterol. 2016; 111:1515.
Article104. Yane K, Kuwatani M, Yoshida M, et al. Non-negligible rate of needle tract seeding after endoscopic ultrasound-guided fine-needle aspiration for patients undergoing distal pancreatectomy for pancreatic cancer. Dig Endosc 2020;32:801–811.105. Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: a learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005; 61:700–708.
Article106. Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004; 59:33–37.
Article107. Harewood GC, Wiersema LM, Halling AC, Keeney GL, Salamao DR, Wiersema MJ. Influence of EUS training and pathology interpretation on accuracy of EUS-guided fine needle aspiration of pancreatic masses. Gastrointest Endosc. 2002; 55:669–673.
Article108. Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001; 54:811–814.
Article109. Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of onsite cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003; 98:1289–1294.
Article110. ASGE Standards of Practice Committee, Faulx AL, Lightdale JR, et al. Guidelines for privileging, credentialing, and proctoring to perform GI endoscopy. Gastrointest Endosc. 2017; 85:273–281.111. ASGE Training Committee, DiMaio CJ, Mishra G, et al. EUS core curriculum. Gastrointest Endosc. 2012; 76:476–481.112. Wani S, Coté GA, Keswani R, et al. Learning curves for EUS by using cumulative sum analysis: implications for American Society for Gastrointestinal Endoscopy recommendations for training. Gastrointest Endosc. 2013; 77:558–565.
Article113. Wani S, Han S, Simon V, et al. Setting minimum standards for training in EUS and ERCP: results from a prospective multicenter study evaluating learning curves and competence among advanced endoscopy trainees. Gastrointest Endosc. 2019; 89:1160–1168.e9.114. Sarker SK, Albrani T, Zaman A, Kumar I. Procedural performance in gastrointestinal endoscopy: live and simulated. World J Surg. 2010; 34:1764–1770.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic ultrasound-guided tissue acquisition: Needle types, technical issues, and sample handling
- Present and Future of Endoscopic Ultrasound-Guided Tissue Acquisition in Solid Pancreatic Tumors
- Endoscopic Ultrasound-Guided Direct Intervention for Solid Pancreatic Tumors
- Technical tips for endoscopic ultrasound-guided pancreatic duct access and drainage
- Clinical and Technical Guideline for Endoscopic Ultrasound (EUS)-Guided Tissue Acquisition of Pancreatic Solid Tumor: Korean Society of Gastrointestinal Endoscopy (KSGE)