Clin Endosc.
2019 Nov;52(6):541-548. 10.5946/ce.2019.127.
Present and Future of Endoscopic Ultrasound-Guided Tissue Acquisition in Solid Pancreatic Tumors
- Affiliations
-
- 1Digestive Disease Center and Research Institute, Department of Internal Medicine, Soon Chun Hyang University School of Medicine, Bucheon, Korea.
- 2Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. lkhyuck@gmail.com
- KMID: 2465796
- DOI: http://doi.org/10.5946/ce.2019.127
Abstract
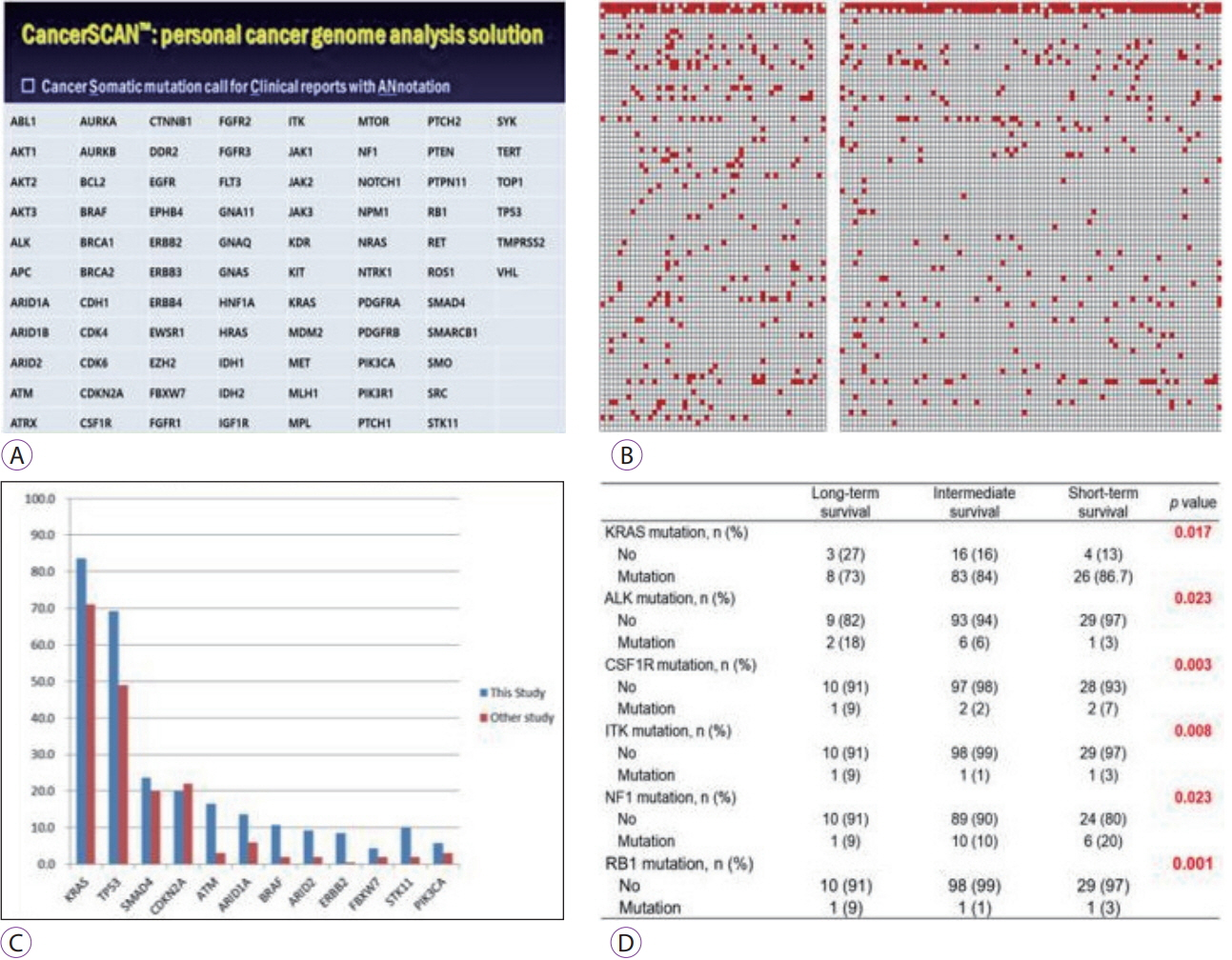
- Endoscopic ultrasound-guided tissue acquisition (EUS-TA) is a well-established method for pathological diagnosis of solid pancreatic neoplasm. It can be performed either as EUS-guided fine-needle aspiration (EUS-FNA) or EUS-guided fine-needle biopsy (EUS-FNB). The incidence of adverse events related to EUS-TA is less than 1%. The factors that affect the diagnostic accuracy and specimen adequacy include the techniques used, type and size of the needle, competency of endosonographers, presence of cytopathologists/cytotechnologists, and rapid on-site examination. EUS-TA may contribute to precision medicine through obtaining tissue samples for next-generation sequencing. The current status, several clinical issues for diagnostic yield and adverse events, and future perspectives of EUS-FNA/FNB for diagnosing pancreatic neoplasm have been discussed in this review article.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee JK, Choi JH, Lee KH, et al. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013; 77:745–751.
Article2. Lee JK, Lee KT, Choi ER, et al. A prospective, randomized trial comparing 25-gauge and 22-gauge needles for endoscopic ultrasound-guided fine needle aspiration of pancreatic masses. Scand J Gastroenterol. 2013; 48:752–757.
Article3. Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2011; 43:897–912.
Article4. Yoshinaga S, Suzuki H, Oda I, Saito Y. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc. 2011; 23 Suppl 1:29–33.
Article5. Iglesias-Garcia J, Lariño-Noia J, Abdulkader I, Domínguez-Muñoz JE. Rapid on-site evaluation of endoscopic-ultrasound-guided fine-needle aspiration diagnosis of pancreatic masses. World J Gastroenterol. 2014; 20:9451–9457.
Article6. Fritscher-Ravens A, Topalidis T, Bobrowski C, et al. Endoscopic ultrasound-guided fine-needle aspiration in focal pancreatic lesions: a prospective intraindividual comparison of two needle assemblies. Endoscopy. 2001; 33:484–490.
Article7. Itoi T, Sofuni A, Itokawa F, Irisawa A, Khor CJ, Rerknimitr R. Current status of diagnostic endoscopic ultrasonography in the evaluation of pancreatic mass lesions. Dig Endosc. 2011; 23 Suppl 1:17–21.
Article8. Eloubeidi MA, Chen VK, Eltoum IA, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003; 98:2663–2668.
Article9. Levy MJ, Wiersema MJ. EUS-guided Trucut biopsy. Gastrointest Endosc. 2005; 62:417–426.
Article10. Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011; 106:1705–1710.
Article11. Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011; 73:1189–1196.
Article12. Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline - updated January 2017. Endoscopy. 2017; 49:695–714.
Article13. Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG clinical guideline: diagnosis and management of pancreatic cysts. Am J Gastroenterol. 2018; 113:464–479.
Article14. ASGE Standards of Practice Committee, Muthusamy VR, Chandrasekhara V, et al. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest Endosc. 2016; 84:1–9.
Article15. Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017; 17:738–753.
Article16. Ang TL, Kwek ABE, Wang LM. Diagnostic endoscopic ultrasound: technique, current status and future directions. Gut Liver. 2018; 12:483–496.
Article17. Ayres LR, Kmiotek EK, Lam E, Telford JJ. A comparison of endoscopic ultrasound-guided fine-needle aspiration and fine-needle biopsy in the diagnosis of solid pancreatic lesions. Can J Gastroenterol Hepatol. 2018; 2018:1415062.
Article18. Noh DH, Choi K, Gu S, et al. Comparison of 22-gauge standard fine needle versus core biopsy needle for endoscopic ultrasound-guided sampling of suspected pancreatic cancer: a randomized crossover trial. Scand J Gastroenterol. 2018; 53:94–99.
Article19. Park JK, Kang KJ, Oh CR, et al. Evaluating the minimal specimens from endoscopic ultrasound-guided fine-needle aspiration in pancreatic masses. Medicine (Baltimore). 2016; 95:e3740.
Article20. Lee YN, Moon JH, Kim HK, et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized parallel-group study. Endoscopy. 2014; 46:1056–1062.
Article21. Vanbiervliet G, Napoléon B, Saint Paul MC, et al. Core needle versus standard needle for endoscopic ultrasound-guided biopsy of solid pancreatic masses: a randomized crossover study. Endoscopy. 2014; 46:1063–1070.
Article22. Strand DS, Jeffus SK, Sauer BG, Wang AY, Stelow EB, Shami VM. EUS-guided 22-gauge fine-needle aspiration versus core biopsy needle in the evaluation of solid pancreatic neoplasms. Diagn Cytopathol. 2014; 42:751–758.
Article23. Bang JY, Hebert-Magee S, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012; 76:321–327.
Article24. Hucl T, Wee E, Anuradha S, et al. Feasibility and efficiency of a new 22G core needle: a prospective comparison study. Endoscopy. 2013; 45:792–798.
Article25. Aadam AA, Wani S, Amick A, et al. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016; 4:E497–E505.
Article26. Kamata K, Kitano M, Yasukawa S, et al. Histologic diagnosis of pancreatic masses using 25-gauge endoscopic ultrasound needles with and without a core trap: a multicenter randomized trial. Endoscopy. 2016; 48:632–638.
Article27. Alatawi A, Beuvon F, Grabar S, et al. Comparison of 22G reverse-beveled versus standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United European Gastroenterol J. 2015; 3:343–352.
Article28. Wang J, Zhao S, Chen Y, Jia R, Zhang X. Endoscopic ultrasound guided fine needle aspiration versus endoscopic ultrasound guided fine needle biopsy in sampling pancreatic masses: a meta-analysis. Medicine (Baltimore). 2017; 96:e7452.29. Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline - March 2017. Endoscopy. 2017; 49:989–1006.
Article30. El Hajj II, Wu H, Reuss S, et al. Prospective assessment of the performance of a new fine needle biopsy device for EUS-guided sampling of solid lesions. Clin Endosc. 2018; 51:576–583.
Article31. Kwon CI. Will new instruments for endoscopic ultrasound-guided tissue acquisition make us happy? Clin Endosc. 2018; 51:510–512.
Article32. Wallace MB, Kennedy T, Durkalski V, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001; 54:441–447.
Article33. Attam R, Arain MA, Bloechl SJ, et al. “Wet suction technique (WEST)”: a novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015; 81:1401–1407.34. Kudo T, Kawakami H, Hayashi T, et al. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014; 80:1030–1037.e1.
Article35. Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018; 87:1432–1438.36. Wani S, Early D, Kunkel J, et al. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: a prospective, single blind, randomized, controlled trial. Gastrointest Endosc. 2012; 76:328–335.
Article37. Kim JH, Park SW, Kim MK, et al. Meta-analysis for cyto-pathological outcomes in endoscopic ultrasonography-guided fine-needle aspiration with and without the stylet. Dig Dis Sci. 2016; 61:2175–2184.
Article38. Abe Y, Kawakami H, Oba K, et al. Effect of a stylet on a histological specimen in EUS-guided fine-needle tissue acquisition by using 22-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2015; 82:837–844.e1.
Article39. Park SW, Chung MJ, Lee SH, et al. Prospective study for comparison of endoscopic ultrasound-guided tissue acquisition using 25- and 22-gauge core biopsy needles in solid pancreatic masses. PLoS One. 2016; 11:e0154401.
Article40. Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001; 54:811–814.
Article41. Shahidi N, Ou G, Lam E, Enns R, Telford J. When trainees reach competency in performing endoscopic ultrasound: a systematic review. Endosc Int Open. 2017; 5:E239–E243.
Article42. Wani S, Keswani R, Hall M, et al. A prospective multicenter study evaluating learning curves and competence in endoscopic ultrasound and endoscopic retrograde cholangiopancreatography among advanced endoscopy trainees: the rapid assessment of trainee endoscopy skills study. Clin Gastroenterol Hepatol. 2017; 15:1758–1767.e11.43. Wani S, Hall M, Keswani RN, et al. Variation in aptitude of trainees in endoscopic ultrasonography, based on cumulative sum analysis. Clin Gastroenterol Hepatol. 2015; 13:1318–1325.e2.
Article44. Wani S, Coté GA, Keswani R, et al. Learning curves for EUS by using cumulative sum analysis: implications for American Society for Gastrointestinal Endoscopy recommendations for training. Gastrointest Endosc. 2013; 77:558–565.
Article45. Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015; 110:1429–1439.
Article46. Rodrigues-Pinto E, Jalaj S, Grimm IS, Baron TH. Impact of EUS-guided fine-needle biopsy sampling with a new core needle on the need for onsite cytopathologic assessment: a preliminary study. Gastrointest Endosc. 2016; 84:1040–1046.
Article47. Keswani RN, Krishnan K, Wani S, Keefer L, Komanduri S. Addition of endoscopic ultrasound (EUS)-guided fine needle aspiration and on-site cytology to EUS-guided fine needle biopsy increases procedure time but not diagnostic accuracy. Clin Endosc. 2014; 47:242–247.
Article48. Schmidt RL, Walker BS, Howard K, Layfield LJ, Adler DG. Rapid onsite evaluation reduces needle passes in endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesions: a risk-benefit analysis. Dig Dis Sci. 2013; 58:3280–3286.
Article49. Wani S, Muthusamy VR, Komanduri S. EUS-guided tissue acquisition: an evidence-based approach (with videos). Gastrointest Endosc. 2014; 80:939–959.e7.
Article50. Wani S, Shah RJ. EUS-guided tissue acquisition: do we need to shoot for a “core” to score? Gastrointest Endosc. 2016; 84:1047–1049.51. Lee KH, Kim EY, Cho J, et al. Risk factors associated with adverse events during endoscopic ultrasound-guided tissue sampling. PLoS One. 2017; 12:e0189347.
Article52. Hamada T, Yasunaga H, Nakai Y, et al. Severe bleeding and perforation are rare complications of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: an analysis of 3,090 patients from 212 hospitals. Gut Liver. 2014; 8:215–218.
Article53. Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011; 73:283–290.
Article54. Yang Y, Li L, Qu C, Liang S, Zeng B, Luo Z. Endoscopic ultrasound-guided fine needle core biopsy for the diagnosis of pancreatic malignant lesions: a systematic review and meta-analysis. Sci Rep. 2016; 6:22978.
Article55. Guan YF, Li GR, Wang RJ, et al. Application of next-generation sequencing in clinical oncology to advance personalized treatment of cancer. Chin J Cancer. 2012; 31:463–470.
Article56. Park JK, Lee JH, Noh DH, et al. Factors of endoscopic ultrasound-guided tissue acquisition for successful next-generation sequencing in pancreatic ductal adenocarcinoma. Gut Liver. 2019. Oct 8 [Epub]. https://doi/org/10.5009/gnl19011.
Article57. Gleeson FC, Kipp BR, Voss JS, et al. Endoscopic ultrasound fine-needle aspiration cytology mutation profiling using targeted next-generation sequencing: personalized care for rectal cancer. Am J Clin Pathol. 2015; 143:879–888.58. Bellevicine C, Vita GD, Malapelle U, Troncone G. Applications and limitations of oncogene mutation testing in clinical cytopathology. Semin Diagn Pathol. 2013; 30:284–297.
Article59. Navina S, McGrath K, Chennat J, et al. Adequacy assessment of endoscopic ultrasound-guided, fine-needle aspirations of pancreatic masses for theranostic studies: optimization of current practices is warranted. Arch Pathol Lab Med. 2014; 138:923–928.
Article60. Roy-Chowdhuri S, Chen H, Singh RR, et al. Concurrent fine needle aspirations and core needle biopsies: a comparative study of substrates for next-generation sequencing in solid organ malignancies. Mod Pathol. 2017; 30:499–508.
Article61. Wei S, Lieberman D, Morrissette JJ, Baloch ZW, Roth DB, McGrath C. Using “residual” FNA rinse and body fluid specimens for next-generation sequencing: an institutional experience. Cancer Cytopathol. 2016; 124:324–329.
Article62. Rekhtman N, Roy-Chowdhuri S. Cytology secimens: a goldmine for molecular testing. Arch Pathol Lab Med. 2016; 140:1189–1190.63. Rodriguez SA, Impey SD, Pelz C, et al. RNA sequencing distinguishes benign from malignant pancreatic lesions sampled by EUS-guided FNA. Gastrointest Endosc. 2016; 84:252–258.
Article64. Bournet B, Gayral M, Torrisani J, Selves J, Cordelier P, Buscail L. Role of endoscopic ultrasound in the molecular diagnosis of pancreatic cancer. World J Gastroenterol. 2014; 20:10758–10768.
Article65. Gayral M, Jo S, Hanoun N, et al. MicroRNAs as emerging biomarkers and therapeutic targets for pancreatic cancer. World J Gastroenterol. 2014; 20:11199–11209.
Article66. Berry W, Algar E, Kumar B, et al. Endoscopic ultrasound-guided fine-needle aspirate-derived preclinical pancreatic cancer models reveal panitumumab sensitivity in KRAS wild-type tumors. Int J Cancer. 2017; 140:2331–2343.67. Ribeiro A, Peng J, Casas C, Fan YS. Endoscopic ultrasound guided fine needle aspiration with fluorescence in situ hybridization analysis in 104 patients with pancreatic mass. J Gastroenterol Hepatol. 2014; 29:1654–1658.
Article68. Boj SF, Hwang CI, Baker LA, Engle DD, Tuveson DA, Clevers H. Model organoids provide new research opportunities for ductal pancreatic cancer. Mol Cell Oncol. 2016; 3:e1014757.
Article69. Boj SF, Hwang CI, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015; 160:324–338.70. Huang L, Holtzinger A, Jagan I, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015; 21:1364–1371.71. Buscaglia JM, Bucobo JC, Tiriac H, et al. Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy (EUSFNB) for personalized cancer treatment. Gastrointest Endosc. 2017; 85(5 Suppl):AB50–AB51.72. Tan SJ, Yeo T, Sukhatme SA, Kong SL, Lim WT, Lim CT. Personalized treatment through detection and monitoring of genetic aberrations in single circulating tumor cells. Adv Exp Med Biol. 2017; 994:255–273.
Article73. Palmirotta R, Lovero D, Silvestris E, et al. Next-generation sequencing (NGS) analysis on single circulating tumor cells (CTCs) with no need of whole-genome amplification (WGA). Cancer Genomics Proteomics. 2017; 14:173–179.
Article74. Ankeny JS, Court CM, Hou S, et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br J Cancer. 2016; 114:1367–1375.
Article75. Catenacci DV, Chapman CG, Xu P, et al. Acquisition of portal venous circulating tumor cells from patients with pancreaticobiliary cancers by endoscopic ultrasound. Gastroenterology. 2015; 149:1794–1803.e4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Technical Advances in Endoscopic Ultrasound (EUS)-Guided Tissue Acquisition for Pancreatic Cancers: How Can We Get the Best Results with EUS-Guided Fine Needle Aspiration?
- Endoscopic ultrasound-guided tissue acquisition: Needle types, technical issues, and sample handling
- Endoscopic Ultrasound-Guided Direct Intervention for Solid Pancreatic Tumors
- Fine-Needle Biopsy: Should This Be the First Choice in Endoscopic Ultrasound-Guided Tissue Acquisition?
- Contrast Harmonic Endoscopic Ultrasound in Pancreatic Diseases