Blood Res.
2021 Mar;56(1):44-46. 10.5045/br.2021.2020184.
Emicizumab prophylaxis in a Korean child with severe hemophilia A and high titer inhibitor: a case report
- Affiliations
-
- 1Department of Pediatrics, Keimyung University School of Medicine, Keimyung University Dongsan Hospital, Daegu, Korea.
- 2Department of Pediatrics, Keimyung University School of Medicine, Keimyung University Daegu Dongsan Hospital, Daegu, Korea.
- KMID: 2514064
- DOI: http://doi.org/10.5045/br.2021.2020184
Abstract
- No abstract available
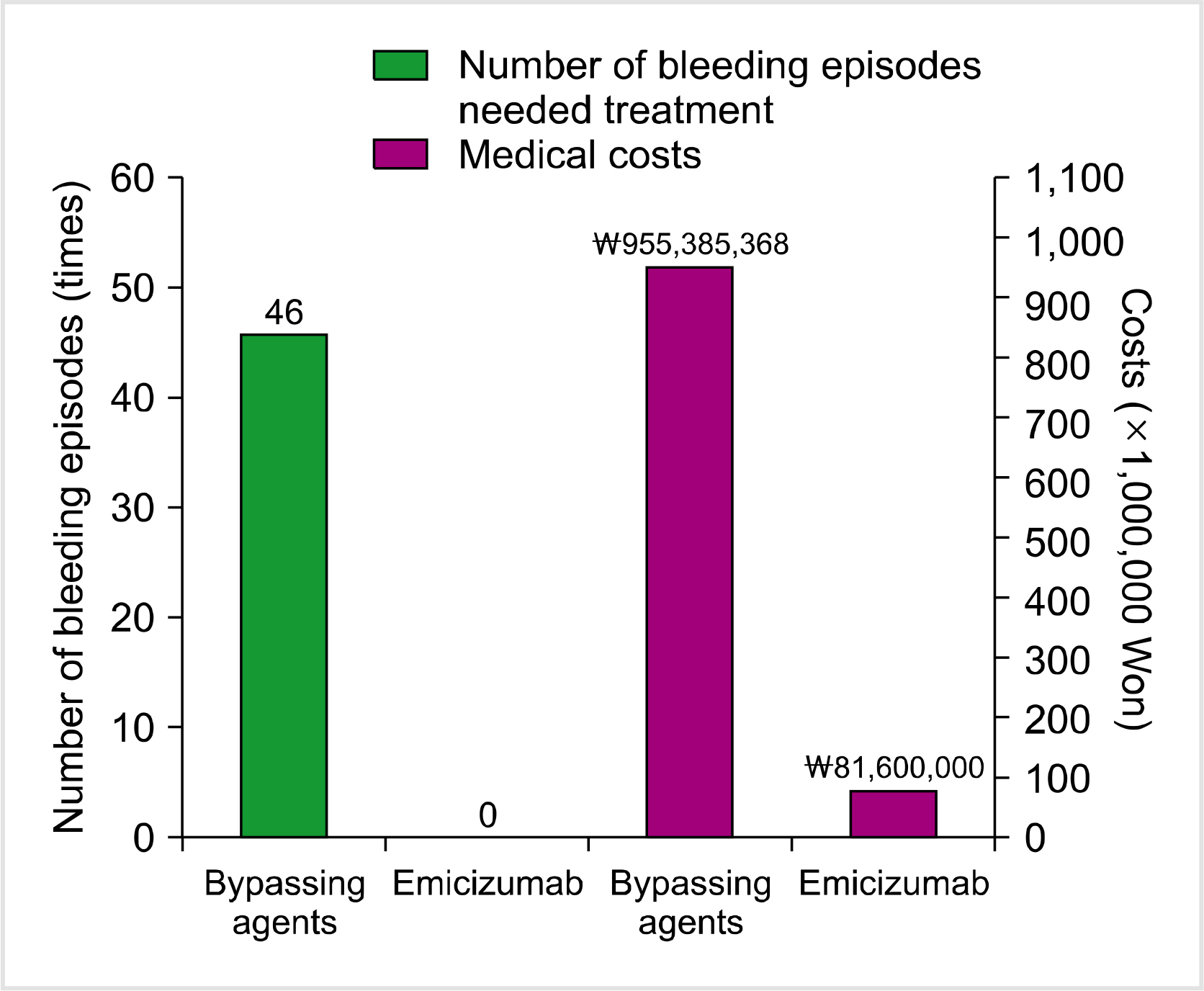
Figure
Reference
-
1. Walsh CE, Soucie JM, Miller CH. United States Hemophilia Treatment Center Network. 2015; Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. 90:400–5. DOI: 10.1002/ajh.23957. PMID: 25616111.2. Cortesi PA, Castaman G, Trifirò G, et al. 2020; Cost-effectiveness and budget impact of emicizumab prophylaxis in haemophilia a patients with inhibitors. Thromb Haemost. 120:216–28. DOI: 10.1055/s-0039-3401822. PMID: 31887777.3. Witmer C, Young G. 2013; Factor VIII inhibitors in hemophilia A: rationale and latest evidence. Ther Adv Hematol. 4:59–72. DOI: 10.1177/2040620712464509. PMID: 23610614. PMCID: PMC3629762.4. Oldenburg J, Mahlangu JN, Kim B, et al. 2017; Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 377:809–18. DOI: 10.1056/NEJMoa1703068. PMID: 28691557.5. D'Angiolella LS, Cortesi PA, Rocino A, et al. 2018; The socioeconomic burden of patients affected by hemophilia with inhibitors. Eur J Haematol. 101:435–56. DOI: 10.1111/ejh.13108. PMID: 29889317.6. Kitazawa T, Igawa T, Sampei Z, et al. 2012; A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 18:1570–4. DOI: 10.1038/nm.2942. PMID: 23023498.7. Mahlangu J, Oldenburg J, Paz-Priel I, et al. 2018; Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 379:811–22. DOI: 10.1056/NEJMoa1803550. PMID: 30157389.8. Shima M, Nogami K, Nagami S, et al. 2019; A multicentre, open-label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors. Haemophilia. 25:979–87. DOI: 10.1111/hae.13848. PMID: 31515851. PMCID: PMC6900083.9. Young G, Liesner R, Chang T, et al. 2019; A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 134:2127–38. DOI: 10.1182/blood.2019001869. PMID: 31697801. PMCID: PMC6908828.10. Gringeri A, Fischer K, Karafoulidou A, et al. 2011; Sequential combined bypassing therapy is safe and effective in the treatment of unresponsive bleeding in adults and children with haemophilia and inhibitors. Haemophilia. 17:630–5. DOI: 10.1111/j.1365-2516.2010.02467.x. PMID: 21323801.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Autoplex Treatment in a Hemophilia A Patient with High Titer of Anticoagulant FVIII Antibody
- A Case of Desensitization for Hemophilia B Inhibitor Patient with Anaphylaxis to FIX Concentrates
- A Case of Secondary Pulmonary Hemosiderosis in a severe Hemophilia A with High Titer of FactorVIII Inhibitor
- Long-term course of anti-factor VIII antibody in patients with hemophilia A at a single center
- The Efficacy of Prophylaxis for Children with Severe Hemophilia in Korea - An Experience of Single Institute