J Korean Med Sci.
2021 Mar;36(10):e71. 10.3346/jkms.2021.36.e71.
Influenza Vaccine Effectiveness in Children at the Emergency Department during the 2018-2019 Season: the First Season School-aged Children Were Included in the Korean Influenza National Immunization Program
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Hospital Medicine, Yongin Severance Hospital, Yonsei University of Medicine, Yongin, Korea
- 3Statistics and Data Center, Samsung Medical Center, Seoul, Korea
- 4Department of Statistics, Keimyung University, Daegu, Korea
- 5Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2513708
- DOI: http://doi.org/10.3346/jkms.2021.36.e71
Abstract
- Background
For the 2018–2019 season, the national influenza immunization program expanded to cover children aged from 6 months to 12 years in Korea. This study aimed to analyze vaccine effectiveness (VE) against influenza in children visiting the pediatric emergency room at a tertiary hospital during the 2018-2019 season.
Methods
Patients tested for influenza antigens from October 1st 2018 to May 31st 2019 at the pediatric emergency room of Samsung Medical Center were included. Patients' influenza antigen test results, influenza vaccination history, and underlying medical conditions were reviewed retrospectively. VE was estimated from the test-negative design study.
Results
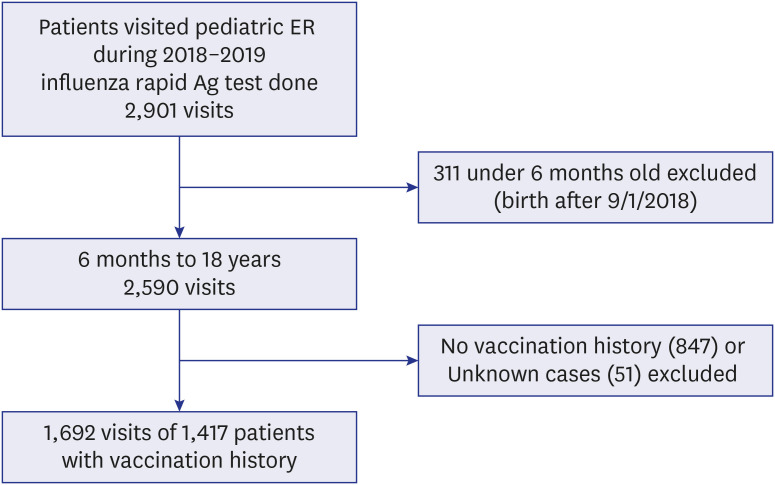
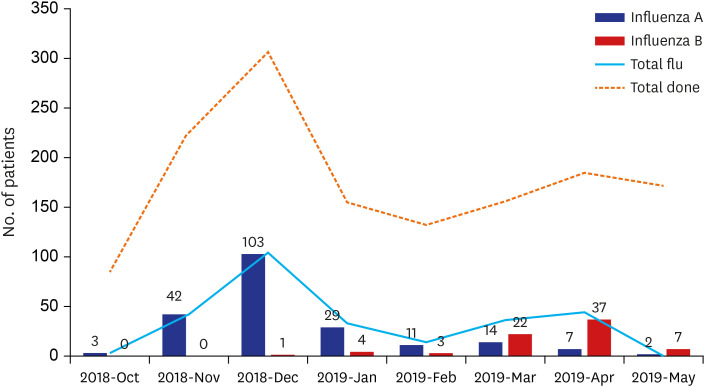
Among the 2,901 visits with influenza test results 1,692 visits of 1,417 patients were included for analysis. Among these 1,417 patients, 285 (20.1%) were positive (influenza A, n = 211, 74.0%; influenza B, n = 74, 26.0%). The VE in all patients was 36.4% (95% confidence interval [CI], 13.9 to 53.1). The VE for influenza A was 37.6% (95% CI, 12.6 to 55.5) and VE for influenza B was 24.0% (−38.5 to 58.3). The VE in the age group 6 months to 12 years was significant with a value of 35.6% (95% CI, 10.5 to 53.7); it was not statistically significant in the age group 13 to 18 years. In a multivariate logistic regression model, patients who received an influenza vaccination were less likely to get influenza infection (OR, 0.6; 95% CI, 0.4 to 0.8; P = 0.001), with significant confounding factors such as age group 13 to 18 years (OR, 0.5; 95% CI, 0.3 to 0.8; P = 0.003) and underlying hematology-oncology disease (OR, 0.3; 95% CI, 0.1 to 0.6; P = 0.002).
Conclusion
We report moderate effectiveness of influenza vaccination in previously healthy children aged from 6 months to 12 years in the 2018-2019 season.
Figure
Cited by 1 articles
-
Delayed Influenza Treatment in Children With False-Negative Rapid Antigen Test: A Retrospective Single-Center Study in Korea 2016–2019
Ji Young Lee, Seung Hwan Baek, Jong Gyun Ahn, Seo Hee Yoon, Moon Kyu Kim, Soo Yeon Kim, Kyung Won Kim, Myung Hyun Sohn, Ji-Man Kang
J Korean Med Sci. 2021;37(1):e3. doi: 10.3346/jkms.2022.37.e3.
Reference
-
1. Centers for Disease Control and Prevention. Influenza historic timeline. Updated 2019. Accessed June 10, 2020. https://www.cdc.gov/flu/pandemic-resources/pandemic-timeline-1930-and-beyond.htm.2. Barberis I, Myles P, Ault SK, Bragazzi NL, Martini M. History and evolution of influenza control through vaccination: from the first monovalent vaccine to universal vaccines. J Prev Med Hyg. 2016; 57(3):E115–20. PMID: 27980374.3. Centers for Disease Control and Prevention. Seasonal influenza vaccine work, vaccine effectiveness studies. Updated 2020. Accessed June 10, 2020. https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html.4. World Health Organization. Influenza fact sheet. Updated 2018. Accessed June 10, 2020. https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal).5. Doyle JD, Chung JR, Kim SS, Gaglani M, Raiyani C, Zimmerman RK, et al. Interim estimates of 2018–19 seasonal influenza vaccine effectiveness—United States, February 2019. MMWR Morb Mortal Wkly Rep. 2019; 68(6):135–139. PMID: 30763298.6. Kissling E, Rose A, Emborg HD, Gherasim A, Pebody R, Pozo F, et al. Interim 2018/19 influenza vaccine effectiveness: six European studies, October 2018 to January 2019. Euro Surveill. 2019; 24(8):1900121.
Article7. Korea Centers for Disease Control and Prevention. 2018–2019 Influenza Management Guideline. Cheongju: Korea Centers for Disease Control and Prevention;2018.8. Yun JW, Noh JY, Song JY, Chun C, Kim Y, Cheong HJ. The Korean influenza national immunization program: history and present status. Infect Chemother. 2017; 49(4):247–254. PMID: 29299891.
Article9. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013; 31(17):2165–2168. PMID: 23499601.
Article10. Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep. 2018; 67(3):1–20.
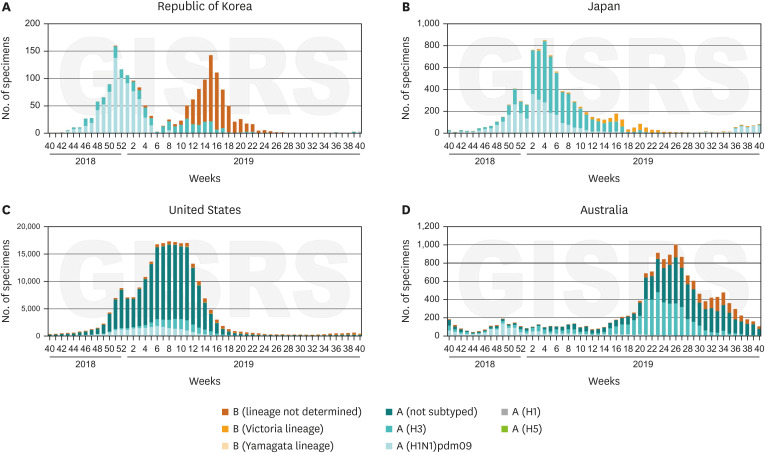
Article11. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2018–2019 northern hemisphere influenza season. Wkly Epidemiol Rec. 2018; 93(12):133–141. PMID: 29569429.12. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2019–2020 northern hemisphere influenza season. Wkly Epidemiol Rec. 2019; 94:141–150.13. Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, et al. Field evaluation of vaccine efficacy. Bull World Health Organ. 1985; 63(6):1055–1068. PMID: 3879673.14. World Health Organization. FluNet-CHARTS of South Korea, USA, Japan, and Australia (from 40th week in 2018 to 40th week in 2019). Updated 2021. Accessed June 10, 2020. http://apps.who.int/flumart/Default?ReportNo=7.15. Jung JE, Lee HR, Choi YH, Kim G. Immunization Program against Influenza in Korea; The 2018–2019 Influenza Season. Cheongju: Korea Centers for Disease Control and Prevention;2019. p. 2241–2249.16. Korea Centers for Disease Control and Prevention. Influenza vaccination rate by age in 2018–2019 season. Updated 2019. Accessed June 10, 2020. http://www.cdc.go.kr/board.es?mid=a20602010000&bid=0034&act=view&list_no=365447.17. Centers for Disease Control and Prevention. Who should and who should not get a flu vaccine. Updated 2020. Accessed October 27, 2020. https://www.cdc.gov/flu/prevent/whoshouldvax.htm.18. Australian Government Department of Health. Flu (influenza) immunisation service. Updated 2020. Accessed June 10, 2020. https://www.health.gov.au/health-topics/immunisation/immunisation-services/flu-influenza-immunisation-service.19. Japan Pediatric Society. Changes in the immunization schedule recommended by the Japan Pediatric Society. Updated 2020. Accessed October 27, 2020. https://www.jpeds.or.jp.20. Park SY, Kim JH, Cho EH, Kim WJ. Establishment of surveillance system and network for the evaluation of influenza vaccine effectiveness and estimation of influenza vaccine effectiveness in preventing influenza. Weekly Health and Disease: KCDC. 2020; 13(18):1207–1216.21. Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013; 31(30):3104–3109. PMID: 23624093.
Article22. Flannery B, Kondor RJ, Chung JR, Gaglani M, Reis M, Zimmerman RK, et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis. 2020; 221(1):8–15. PMID: 31665373.
Article23. Park KS, Park SJ, Kwon DH, Lee DH. Influenza Sentinel Surveillance Report in the Republic of Korea, 2018–2019. Public Health Wkly Rep. 2019; 12(49):2224–2232.24. Hammond A, Hundal K, Laurenson-Schafer H, Cozza V, Maharjan B, Fitzner J, et al. Review of the 2018–2019 influenza season in the northern hemisphere. Wkly Epidemiol Rec. 2019; 94(32):345–365.25. Kim HM, Lee NJ, Kim MS, Park JH, Han MG. Korea influenza laboratory surveillance report in 2018–2019 season. Public Health Wkly Rep. 2019; 12(49):2238–2240.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiological Characteristics of Influenza in Children during the 2017– 2018 and 2018–2019 Influenza Seasons in Jeju, Korea
- Factors Associated with the 2017–2018 Seasonal Influenza Vaccination Coverage among Elementary, Middle, and High School Students
- The Pattern of Vaccine Administration Accessed by Vaccine Consumption in a University Hospital
- Seasonal influenza and vaccine herd effect
- The Korean Influenza National Immunization Program: History and Present Status