Estimating Baseline Incidence of Conditions Potentially Associated with Vaccine Adverse Events: a Call for Surveillance System Using the Korean National Health Insurance Claims Data
- Affiliations
-
- 1Division of Infectious Diseases, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Big Data Strategy, National Health Insurance Service, Wonju, Korea
- 3Artificial Intelligence and Big-Data Convergence Center, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 4Department of Internal Medicine, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 5Department of Preventive Medicine, Gachon University College of Medicine, Incheon, Korea
- KMID: 2513587
- DOI: http://doi.org/10.3346/jkms.2021.36.e67
Abstract
- Background
Vaccines against coronavirus disease 2019 (COVID-19) are raising concerns about vaccine safety, particularly in the context of large-scale immunization. To address public concerns, we measured the baseline incidence rates of major conditions potentially related to vaccine-related adverse events (VAEs). We aimed to provide a basis for evaluating VAEs and verifying causality.
Methods
Conditions of interest were selected from the US Vaccine Adverse Event Reporting System Table of Reportable Events and a recent report from a European consortium on vaccine surveillance. We used the National Health Insurance Service database in Korea to identify the monthly numbers of cases with these conditions. Data from January 2006 to June 2020 were included. Prediction models were constructed from the observed incidences using an autoregressive integrated moving average. We predicted the incidences of the conditions and their respective 95% confidence intervals (CIs) for January through December 2021. In addition, subgroup analysis for the expected vaccination population was conducted.
Results
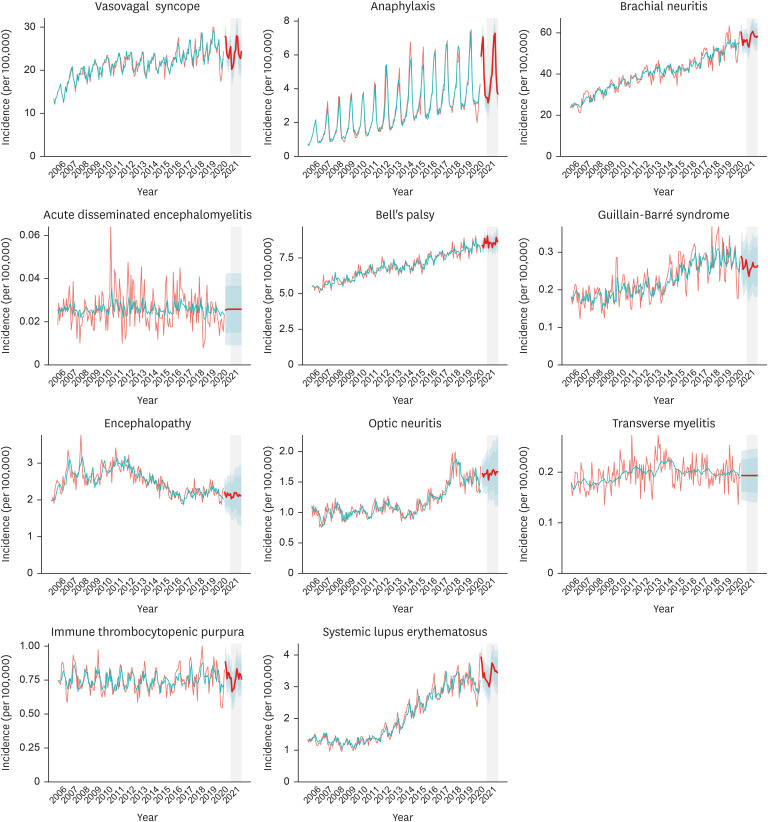
Mean values (95% CIs) of the predicted monthly incidence of vasovagal syncope, anaphylaxis, brachial neuritis, acute disseminated encephalomyelitis, Bell's palsy, Guillain-Barré syndrome, encephalopathy, optic neuritis, transverse myelitis, immune thrombocytopenic purpura, and systemic lupus erythematosus in 2021 were 23.89 (19.81– 27.98), 4.72 (3.83–5.61), 57.62 (51.37–63.88), 0.03 (0.01–0.04), 8.58 (7.90–9.26), 0.26 (0.18– 0.34), 2.13 (1.42–2.83), 1.65 (1.17–2.13), 0.19 (0.14–0.25), 0.75 (0.61–0.90), and 3.40 (2.79– 4.01) cases per 100,000 respectively. The majority of the conditions showed an increasing trend with seasonal variations in their incidences.
Conclusion
We measured the incidence of a total of 11 conditions that could potentially be associated with VAEs to predict the monthly incidence in 2021. In Korea, conditions that could potentially be related to VAEs occur on a regular basis, and an increasing trend is observed with seasonality.
Figure
Cited by 3 articles
-
Adverse Events Following Immunization Associated with Coronavirus Disease 2019 Vaccination Reported in the Mobile Vaccine Adverse Events Reporting System
Minji Jeon, Jehun Kim, Chi Eun Oh, Jin-Young Lee
J Korean Med Sci. 2021;36(17):e114. doi: 10.3346/jkms.2021.36.e114.Predicted and Observed Incidence of Thromboembolic Events among Koreans Vaccinated with ChAdOx1 nCoV-19 Vaccine
Kyungmin Huh, Yewon Na, Young-Eun Kim, Munkhzul Radnaabaatar, Kyong Ran Peck, Jaehun Jung
J Korean Med Sci. 2021;36(27):e197. doi: 10.3346/jkms.2021.36.e197.Machine Learning Approach for Active Vaccine Safety Monitoring
Yujeong Kim, Jong-Hwan Jang, Namgi Park, Na-Young Jeong, Eunsun Lim, Soyun Kim, Nam-Kyong Choi, Dukyong Yoon
J Korean Med Sci. 2021;36(31):e198. doi: 10.3346/jkms.2021.36.e198.
Reference
-
1. Korea Centers for Disease Control & Prevention. Free vaccination for All Citizens of COVID-19 to Return to Daily Life. Cheongju: Korea Centers for Disease Control & Prevention;2021.2. Jung J. Epidemiologic evaluation and risk communication regarding the recent reports of sudden death after influenza vaccination in the COVID-19 pandemic. J Korean Med Sci. 2020; 35(41):e378. PMID: 33107233.
Article3. CDC COVID-19 Response Team. Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021; 70(2):46–51. PMID: 33444297.4. Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397(10269):99–111. PMID: 33306989.5. U.S. Department of Health and Human Services. Vaccine adverse event reporting system (VAERS) table of reportable events following vaccination. Updated 2021. Accessed January 28, 2021. https://vaers.hhs.gov/docs/VAERS_Table_of_Reportable_Events_Following_Vaccination.pdf.6. Willame C, Dodd C, van der Aa L, Picelli G, Emborg HD, Kahlert J, et al. Incidence rates of autoimmune diseases in european healthcare databases: a contribution of the ADVANCE project. Drug Saf. 2021; 44(3):383–395. PMID: 33462778.
Article7. Hyndman R, Athanasopoulos G, Bergmeir C, Caceres G, Chhay L, O'Hara-Wild M, et al. Forecasting functions for time series and linear models. Updated 2021. Accessed January 28, 2021. https://pkg.robjhyndman.com/forecast/.8. Baker MA, Nguyen M, Cole DV, Lee GM, Lieu TA. Post-licensure rapid immunization safety monitoring program (PRISM) data characterization. Vaccine. 2013; 31(Suppl 10):K98–K112. PMID: 24331080.
Article9. Yang MS, Kim JY, Kim BK, Park HW, Cho SH, Min KU, et al. True rise in anaphylaxis incidence: epidemiologic study based on a national health insurance database. Medicine (Baltimore). 2017; 96(5):e5750. PMID: 28151851.10. Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. Forthcoming 2021. DOI: 10.1001/jama.2021.1967.
Article11. GOV.UK. Coronavirus (COVID-19) vaccine adverse reactions. Updated 2021. Accessed February 12, 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions.12. Yoon HH, Park JY, Kim SY, Lee NM, Yi DY, Yun SW, et al. Epidemiology of demyelinating diseases in Korean pediatric patients. J Child Neurol. 2021; 36(2):141–147. PMID: 32988277.
Article13. Kim C, Rhie S, Suh M, Kang DR, Choi YJ, Bae GR, et al. Pandemic influenza A vaccination and incidence of Guillain-Barré syndrome in Korea. Vaccine. 2015; 33(15):1815–1823. PMID: 25728315.
Article14. Lee JY, Han J, Yang M, Oh SY. Population-based incidence of pediatric and adult optic neuritis and the risk of multiple sclerosis. Ophthalmology. 2020; 127(3):417–425. PMID: 31732227.
Article15. Lee JY, Lee JH, Lee H, Kang B, Kim JW, Kim SH, et al. Epidemiology and management of primary immune thrombocytopenia: a nationwide population-based study in Korea. Thromb Res. 2017; 155:86–91. PMID: 28525829.
Article16. Bae EH, Lim SY, Han KD, Jung JH, Choi HS, Kim HY, et al. Trend of prevalence and incidence of systemic lupus erythematosus in South Korea, 2005 to 2015: a nationwide population-based study. Korean J Intern Med. 2020; 35(3):652–661. PMID: 31212409.
Article17. McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016; 137(3):868–878. PMID: 26452420.
Article18. Gee J, Sukumaran L, Weintraub E. Vaccine Safety Datalink Team. Risk of Guillain-Barré Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. Vaccine. 2017; 35(43):5756–5758. PMID: 28935469.
Article19. CDC. V-safe after vaccination health checker. Updated 2021. Accessed February 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html.20. MHRA. Yellow card. Updated 2021. Accessed February 12, 2021. https://yellowcard.mhra.gov.uk/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- JYNNEOS vaccine safety monitoring in the Republic of Korea, 2022: a cross-sectional study
- Surveillance and compensation claims for adverse events following immunization from 2011 to 2016 in the Republic of Korea
- Clinical Study Using Healthcare Claims Database
- Sensitivity of Medical Insurance Claims Data Using Population-based Cancer Registry Data
- Machine Learning Approach for Active Vaccine Safety Monitoring