Korean Circ J.
2021 Mar;51(3):251-262. 10.4070/kcj.2020.0303.
Sodium/glucose Co-Transporter 2 Inhibitor, Empagliflozin, Alleviated Transient Expression of SGLT2 after Myocardial Infarction
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea
- 3Department of Physiology, School of Medicine, Pusan National University, Yangsan, Korea
- 4Department of Convergence Medicine, Pusan National University School of Medicine, Yangsan, Korea
- 5Department of Chemistry, Center for Proteome Biophysics and Chemistry Institute for Functional Materials, Pusan National University, Busan, Korea
- 6PNU GRAND Convergence Medical Science Education Research Center, Pusan National University School of Medicine, Yangsan, Korea
- KMID: 2513523
- DOI: http://doi.org/10.4070/kcj.2020.0303
Abstract
- Background and Objectives
Large clinical studies of sodium/glucose cotransporter 2 (SGLT2) inhibitors have shown a significant beneficial effect on heart failure-associated hospitalization and cardiovascular events. As SGLT2 is known to be absent in heart cells, improved cardiovascular outcomes are thought to be accounted for by the indirect effects of the drug. We sought to confirm whether such benefits were mediated through SGLT2 expressed in the heart using myocardial infarction (MI) model.
Methods
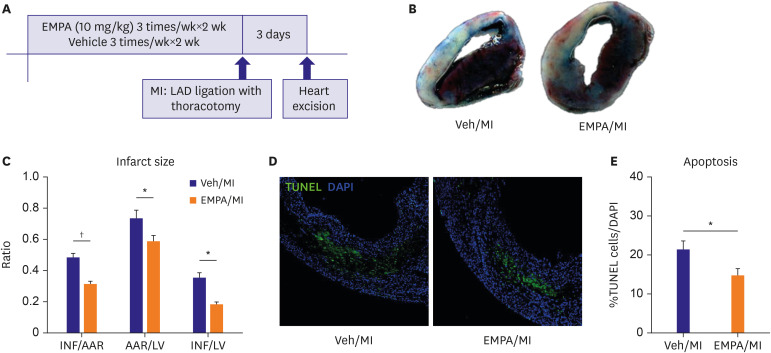
Mice pre-treated with empagliflozin (EMPA), an SGLT2 inhibitor, showed a significantly reduced infarct size compared with the vehicle group three days post-MI. Interestingly, we confirmed SGLT2 localized in the infarct zone. The sequential changes of SGLT2 expression after MI were also evaluated.
Results
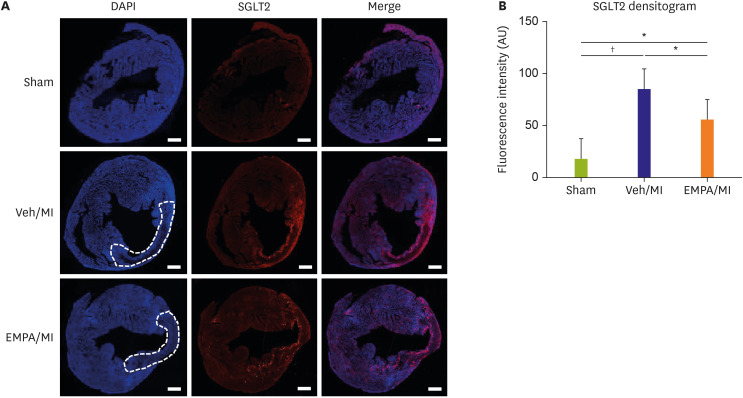
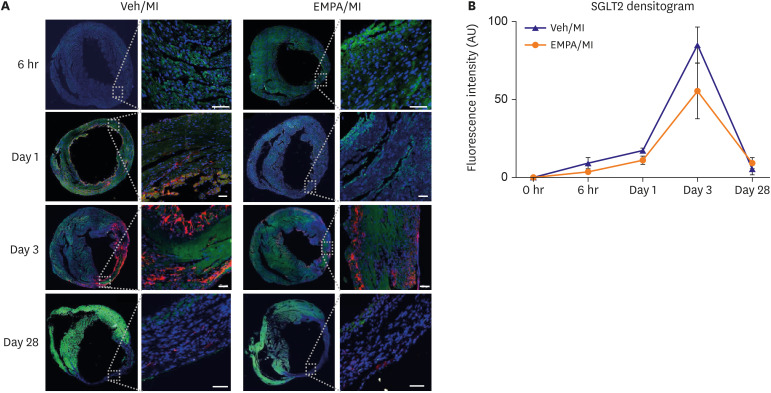
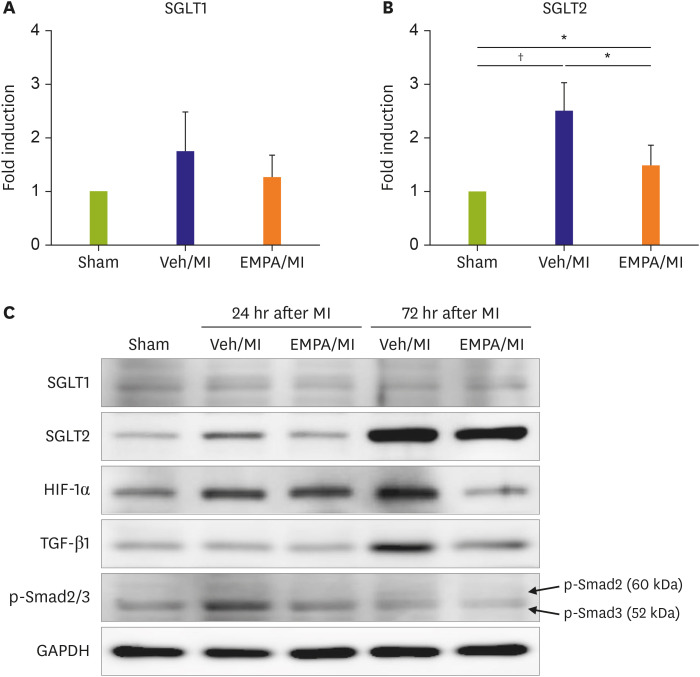
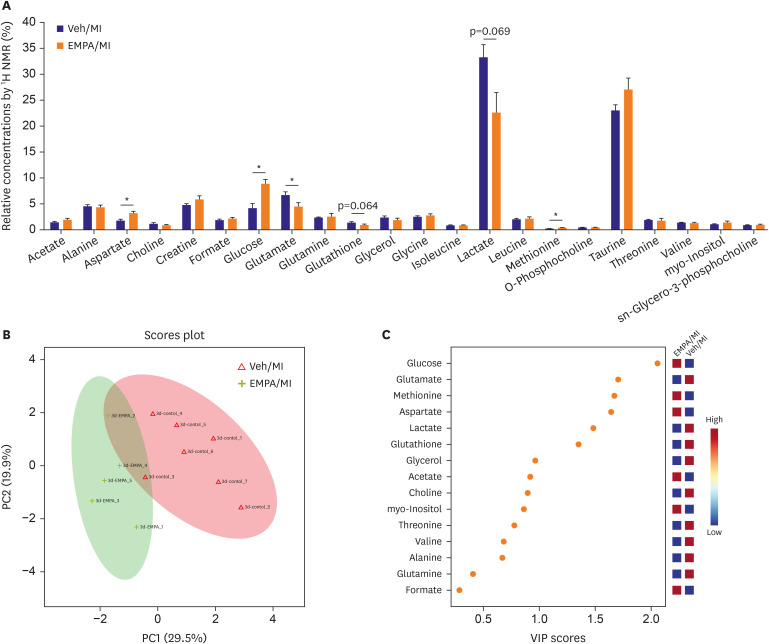
One day after MI, SGLT2 transiently appeared in the ischemic areas in the vehicle group and increased until 72 hours. The appearance of SGLT2 was delayed and less in amount compared with the vehicle group. Additionally, there was a significant difference in metabolites, including glucose and amino acids in the 1 H nuclear magnetic resonance analysis between groups.
Conclusions
Our work demonstrates that SGLT2 is transiently expressed in heart tissue early after MI and EMPA may directly operate on SGLT2 to facilitate metabolic substrates shifts.
Keyword
Figure
Reference
-
1. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–357. PMID: 30415602.
Article2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128. PMID: 26378978.
Article3. Arnott C, Li Q, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020; 9:e014908. PMID: 31992158.
Article4. McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008. PMID: 31535829.5. Damman K, Beusekamp JC, Boorsma EM, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020; 22:713–722. PMID: 31912605.
Article6. Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015; 66:255–270. PMID: 25341005.
Article7. Chen J, Williams S, Ho S, et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther. 2010; 1:57–92. PMID: 22127746.
Article8. Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015; 38:1730–1735. PMID: 26180105.
Article9. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–657. PMID: 28605608.
Article10. Uthman L, Baartscheer A, Schumacher CA, et al. Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol. 2018; 9:1575. PMID: 30519189.
Article11. Ndibalema AR, Kabuye D, Wen S, Li L, Li X, Fan Q. Empagliflozin protects against proximal renal tubular cell injury induced by high glucose via regulation of hypoxia-inducible factor 1-alpha. Diabetes Metab Syndr Obes. 2020; 13:1953–1967. PMID: 32606855.12. Zapata-Morales JR, Galicia-Cruz OG, Franco M, Martinez Y Morales F. Hypoxia-inducible factor-1α (HIF-1α) protein diminishes sodium glucose transport 1 (SGLT1) and SGLT2 protein expression in renal epithelial tubular cells (LLC-PK1) under hypoxia. J Biol Chem. 2014; 289:346–357. PMID: 24196951.
Article13. Panchapakesan U, Pegg K, Gross S, et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells--renoprotection in diabetic nephropathy? PLoS One. 2013; 8:e54442. PMID: 23390498.
Article14. Yurista SR, Silljé HH, Oberdorf-Maass SU, et al. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail. 2019; 21:862–873. PMID: 31033127.
Article15. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007; 261:32–43. PMID: 17222166.
Article16. Zhou L, Cryan EV, D'Andrea MR, Belkowski S, Conway BR, Demarest KT. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem. 2003; 90:339–346. PMID: 14505350.
Article17. Tazawa S, Yamato T, Fujikura H, et al. SLC5A9/SGLT4, a new Na+-dependent glucose transporter, is an essential transporter for mannose, 1,5-anhydro-D-glucitol, and fructose. Life Sci. 2005; 76:1039–1050. PMID: 15607332.
Article18. Banerjee SK, McGaffin KR, Pastor-Soler NM, Ahmad F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res. 2009; 84:111–118. PMID: 19509029.
Article19. Di Franco A, Cantini G, Tani A, et al. Sodium-dependent glucose transporters (SGLT) in human ischemic heart: a new potential pharmacological target. Int J Cardiol. 2017; 243:86–90. PMID: 28526540.
Article20. Lambert R, Srodulski S, Peng X, Margulies KB, Despa F, Despa S. Intracellular Na+ concentration ([Na+]i) is elevated in diabetic hearts due to enhanced Na+-glucose cotransport. J Am Heart Assoc. 2015; 4:e002183. PMID: 26316524.
Article21. Van Steenbergen A, Balteau M, Ginion A, et al. Sodium-myoinositol cotransporter-1, SMIT1, mediates the production of reactive oxygen species induced by hyperglycemia in the heart. Sci Rep. 2017; 7:41166. PMID: 28128227.
Article22. Vrhovac I, Balen Eror D, Klessen D, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015; 467:1881–1898. PMID: 25304002.
Article23. Kashiwagi Y, Nagoshi T, Yoshino T, et al. Expression of SGLT1 in human hearts and impairment of cardiac glucose uptake by phlorizin during ischemia-reperfusion injury in mice. PLoS One. 2015; 10:e0130605. PMID: 26121582.
Article24. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016; 39:1108–1114. PMID: 27289126.
Article25. Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005; 85:1093–1129. PMID: 15987803.
Article26. Taegtmeyer H, Goodwin GW, Doenst T, Frazier OH. Substrate metabolism as a determinant for postischemic functional recovery of the heart. Am J Cardiol. 1997; 80:3A–10A.
Article27. Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994; 19:59–113. PMID: 8174388.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitor
- Sodium-Glucose Cotransporter 2 Inhibitors: Mechanisms of Action and Various Effects
- Fasting is not always good: perioperative fasting leads to pronounced ketone body production in patients treated with SGLT2 inhibitors: a case report
- Glucose Lowering Effect of SGLT2 Inhibitors: A Review of Clinical Studies
- Effect of Empagliflozin, a Selective Sodium-Glucose Cotransporter 2 Inhibitor, on Kidney and Peripheral Nerves in Streptozotocin-Induced Diabetic Rats