Neurointervention.
2021 Mar;16(1):39-45. 10.5469/neuroint.2020.00353.
Metagenomics Analysis of Thrombus Samples Retrieved from Mechanical Thrombectomy
- Affiliations
-
- 1Department of Neurology, Pacific Medical College & Hospital, Pacific Medical University, Udaipur, India
- 2CSIR National Botanical Research Institute, Lucknow, India
- KMID: 2513342
- DOI: http://doi.org/10.5469/neuroint.2020.00353
Abstract
- Purpose
The purpose of this study was to assess the microbiota in middle cerebral artery thrombi retrieved in mechanical thrombectomy arising out of symptomatic carotid plaque within 6 hours of acute ischemic stroke. Thrombi were subjected to next-generation sequencing for a bacterial signature to determine their role in atherosclerosis.
Materials and Methods
We included 4 human middle cerebral artery thrombus samples (all patients were male). The median age for the patients was 51±13.6 years. Patients enrolled in the study from Pacific Medical University and Hospital underwent mechanical thrombectomy in the stroke window period. All patients underwent brain magnetic resonance angiography (MRA) and circle of Willis and neck vessel MRA along with the standard stroke workup to establish stroke etiology. Only patients with symptomatic carotid stenosis and tandem lesions with ipsilateral middle cerebral artery occlusion were included in the study. Thrombus samples were collected, stored at –80 degrees, and subjected to metagenomics analysis.
Results
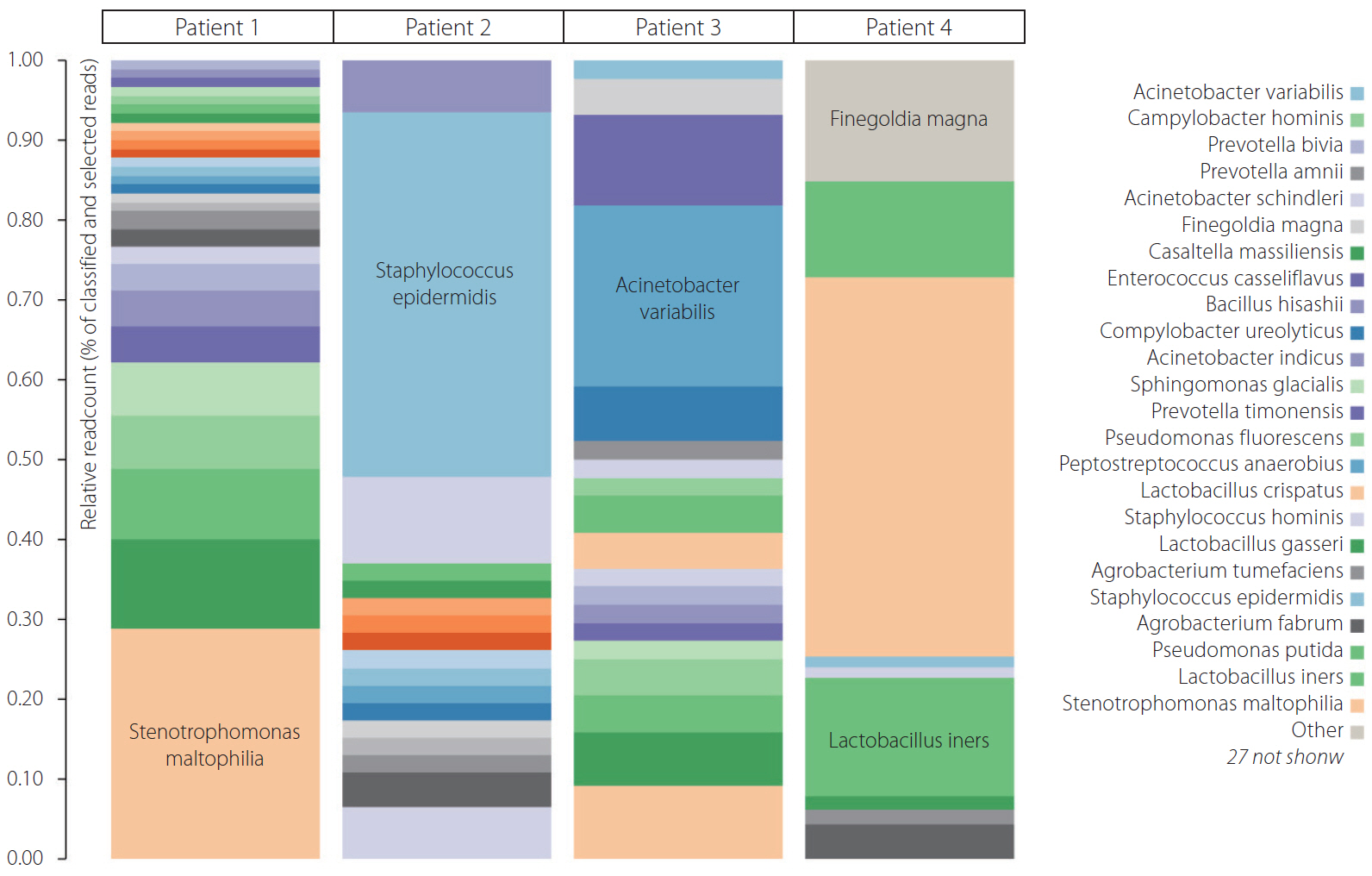
Of the 4 patients undergoing thrombectomy for diagnosis with ischemic stroke, all thrombi recovered for bacterial DNA in qPCR were positive. More than 27 bacteria were present in the 4 thrombus samples. The majority of bacteria were Lactobacillus, Stenotrophomonas, Pseudomonas, Staphylococcus, and Finegoldia.
Conclusion
Genesis of symptomatic atherosclerotic carotid plaque leading to thromboembolism could be either due to direct mechanisms like acidification and local inflammation of plaque milieu with lactobacillus, biofilm dispersion leading to inflammation like with pseudomonas fluorescence, or enterococci or indirect mechanisms like Toll 2 like signaling by gut microbiota.
Keyword
Figure
Cited by 1 articles
-
Clot Composition Analysis as a Diagnostic Tool to Gain Insight into Ischemic Stroke Etiology: A Systematic Review
Alicia Aliena-Valero, Júlia Baixauli-Martín, Germán Torregrosa, José I. Tembl, Juan B. Salom
J Stroke. 2021;23(3):327-342. doi: 10.5853/jos.2021.02306.
Reference
-
1. Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: a global response is needed. Bull World Health Organ. 2016; 94:634–634. A.
Article2. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016; 14:e1002533.
Article3. Epstein SE, Zhu J, Burnett MS, Zhou YF, Vercellotti G, Hajjar D. Infection and atherosclerosis: potential roles of pathogen burden and molecular mimicry. Arterioscler Thromb Vasc Biol. 2000; 20:1417–1420.4. Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997; 96:4095–4103.5. Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011; 106:858–867.
Article6. Lanter BB, Sauer K, Davies DG. Bacteria present in carotid arterial plaques are found as biofilm deposits which may contribute to enhanced risk of plaque rupture. mBio. 2014; 5:e01206–14.
Article7. Amar J, Serino M, Lange C, Chabo C, Iacovoni J, Mondot S, D.E.S.I.R. Study Group, et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011; 54:3055–3061.
Article8. Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011; 108(Supplement 1):4592–4598.
Article9. Yilmaz A, Lipfert B, Cicha I, Schubert K, Klein M, Raithel D, et al. Accumulation of immune cells and high expression of chemokines/chemokine receptors in the upstream shoulder of atherosclerotic carotid plaques. Exp Mol Pathol. 2007; 82:245–255.
Article10. Fagerberg B, Ryndel M, Kjelldahl J, Akyürek LM, Rosengren L, Karlström L, et al. Differences in lesion severity and cellular composition between in vivo assessed upstream and downstream sides of human symptomatic carotid atherosclerotic plaques. J Vasc Res. 2010; 47:221–230.
Article11. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019; 20:6008.
Article12. Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995; 92:657–671.
Article13. Loria V, Cosentino N, Montone RA, Niccoli G. Biomarkers and coronary atherosclerotic burden and activity as assessed by coronary angiography and intra-coronary imaging modalities. In : Branislav B, editor. London: IntechOpen;2011.14. Aziz M, Yadav KS. Pathogenesis of atherosclerosis a review. Med Clin Rev. 2016; 2:22.
Article15. Mughal MM, Khan MK, DeMarco JK, Majid A, Shamoun F, Abela GS. Symptomatic and asymptomatic carotid artery plaque. Expert Rev Cardiovasc Ther. 2011; 9:1315–1330.
Article16. Sauer K, Cullen MC, Rickard AH, Zeef LA, Davies DG, Gilbert P. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J Bacteriol. 2004; 186:7312–7326.17. Mitsuoka T. The human gastrointestinal tract. In : Wood BJB, editor. Lactic acid bacteria in health and disease. Boston: Springer;1992. p. 69–114.18. Husni RN, Gordon SM, Washington JA, Longworth DL. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin Infect Dis. 1997; 25:1048–1055.
Article19. Leake DS. Does an acidic pH explain why low density lipoprotein is oxidised in atherosclerotic lesions? Atherosclerosis. 1997; 129:149–157.
Article20. Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018; 10:1398.
Article21. Chen S, Lee Y, Crother TR, Fishbein M, Zhang W, Yilmaz A, et al. Marked acceleration of atherosclerosis after Lactobacillus casei-induced coronary arteritis in a mouse model of Kawasaki disease. Arterioscler Thromb Vasc Biol. 2012; 32:e60–e71.
Article22. Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012; 53:100–108.
Article23. Lusis AJ. Atherosclerosis. Nature. 2000; 407:233–241.
Article24. Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006; 47(8 Suppl):C7–C12.
Article25. McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol. 2011; 10:39–50.
Article26. Boyd A, Chakrabarty AM. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl Environ Microbiol. 1994; 60:2355–2359.
Article27. Shaban A, Hymel B, Chavez-Keatts M, Karlitz JJ, Martin-Schild S. Recurrent posterior strokes in inflammatory bowel disease patients. Gastroenterol Res Pract. 2015; 2015:672460.
Article28. Flores-Treviño S, Bocanegra-Ibarias P, Camacho-Ortiz A, Morfín-Otero R, Salazar-Sesatty HA, Garza-González E. Stenotrophomonas maltophilia biofilm: its role in infectious diseases. Expert Rev Anti Infect Ther. 2019; 17:877–893.
Article29. Looney WJ, Narita M, Mühlemann K. Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect Dis. 2009; 9:312–323.
Article30. Ford PJ, Gemmell E, Chan A, Carter CL, Walker PJ, Bird PS, et al. Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol Immunol. 2006; 21:206–211.
Article31. Pyysalo MJ, Pyysalo LM, Pessi T, Karhunen PJ, Öhman JE. The connection between ruptured cerebral aneurysms and odontogenic bacteria. J Neurol Neurosurg Psychiatry. 2013; 84:1214–1218.
Article32. Elkaïm R, Dahan M, Kocgozlu L, Werner S, Kanter D, Kretz JG, et al. Prevalence of periodontal pathogens in subgingival lesions, atherosclerotic plaques and healthy blood vessels: a preliminary study. J Periodontal Res. 2008; 43:224–231.
Article33. Renko J, Lepp PW, Oksala N, Nikkari S, Nikkari ST. Bacterial signatures in atherosclerotic lesions represent human commensals and pathogens. Atherosclerosis. 2008; 201:192–197.
Article34. Gilad J, Borer A, Peled N, Riesenberg K, Tager S, Appelbaum A, et al. Hospital-acquired brevundimonas vesicularis septicaemia following open-heart surgery: case report and literature review. Scand J Infect Dis. 2000; 32:90–91.35. Jäckel S, Kiouptsi K, Lillich M, Hendrikx T, Khandagale A, Kollar B, et al. Gut microbiota regulate hepatic von Willebrand factor synthesis and arterial thrombus formation via Toll-like receptor-2. Blood. 2017; 130:542–553.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Real-Time Visualization of Thrombus during Suction Thrombectomy : Contrast-in-Stasis Technique
- Mechanical Thrombectomy for Acute Ischemic Stroke due to Thrombus in the Pulmonary Vein Stump after Left Pulmonary Lobectomy: A Case Series
- Traditional Thrombus Composition and Related Endovascular Outcomes: Catching up with the Recent Evidence
- Suction thrombectomy of distal medium vessel occlusion using microcatheter during mechanical thrombectomy for acute ischemic stroke: A case series
- Comparative Viral Metagenomics of Environmental Samples from Korea