Int J Stem Cells.
2021 Feb;14(1):74-84. 10.15283/ijsc20094.
Human Stem Cell-Derived Retinal Pigment Epithelial Cells as a Model for Drug Screening and Pre-Clinical Assays Compared to ARPE-19 Cell Line
- Affiliations
-
- 1Department of Biochemistry and Immunology, Federal University of Minas Gerais, Belo Horizonte, Brazil
- 2Faculty of Pharmacy, Federal University of Minas Gerais, Belo Horizonte, Brazil
- 3SENAN, Centro de Desenvolvimento da Tecnologia Nuclear – CDTN/CNEN, Federal University of Minas Gerais, Belo Horizonte, Brazil
- 4Department of Genomic Sciences and Biotechnology, Catholic University of Brasília, Brasília, Brazil
- 5Department of Ophthalmology, Federal University of Minas Gerais, Belo Horizonte, Brazil
- 6Pharmaceutical Research and Development, Ezequiel Dias Foundation, Belo Horizonte, Brazil
- KMID: 2513084
- DOI: http://doi.org/10.15283/ijsc20094
Abstract
- Background and Objectives
Eye diseases have a high socioeconomic impact on society and may be one of the fields in which most stem cell-related scientific accomplishments have been achieved recently. In this context, human Pluripotent Stem Cell (hPSC) technology arises as an important tool to produce and study human Embryonic Stem cell derived-Retinal Pigmented Epithelial Cells (hES-RPE) for several applications, such as cell therapy, disease modeling, and drug screening. The use of this technology in pre-clinical phases attends to the overall population desire for animal-free product development. Here, we aimed to compare hES-RPE cells with ARPE-19, one of the most commonly used retinal pigmented epithelial immortalized cell lines.
Methods and Results
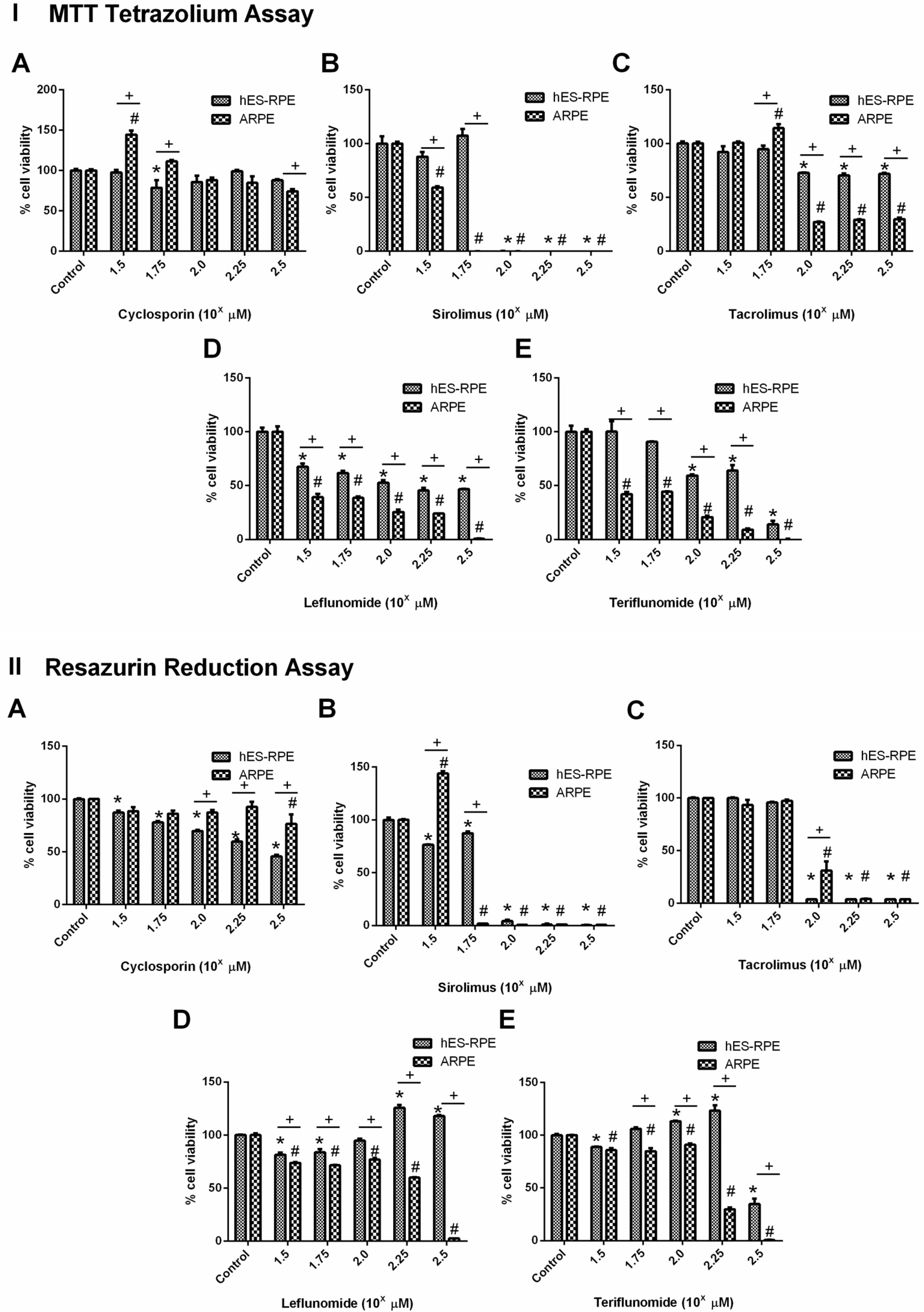
Functional, cellular and molecular data obtained suggest that hES-RPE cells more closely resembles native RPEs compared to ARPE-19. Furthermore, hES-RPE revealed an interesting robustness when cultured on human Bruch’s membrane explants and after exposure to Cyclosporine (CSA), Sirolimus (SRL), Tacrolimus (TAC), Leflunomide (LEF) and Teriflunomide (TER). On these conditions, hES-RPE cells were able to survive at higher drug concentrations, while ARPE-19 cell line was more susceptible to cell death.
Conclusions
Therefore, hES-RPEs seem to have the ability to incur a broader range of RPE functions than ARPE-19 and should be more thoroughly explored for drug screening.
Keyword
Figure
Reference
-
References
1. Wilson SL, Ahearne M, Hopkinson A. 2015; An overview of current techniques for ocular toxicity testing. Toxicology. 327:32–46. DOI: 10.1016/j.tox.2014.11.003. PMID: 25445805.
Article2. Aberdam E, Petit I, Sangari L, Aberdam D. 2017; Induced pluripotent stem cell-derived limbal epithelial cells (LiPSC) as a cellular alternative for in vitro ocular toxicity testing. PLoS One. 12:e0179913. DOI: 10.1371/journal.pone.0179913. PMID: 28640863. PMCID: PMC5481014.
Article3. del Amo EM, Vellonen KS, Kidron H, Urtti A. 2015; Intravitreal clearance and volume of distribution of compounds in rabbits: In silico prediction and pharmacokinetic simulations for drug development. Eur J Pharm Biopharm. 95(Pt B):215–226. DOI: 10.1016/j.ejpb.2015.01.003. PMID: 25603198.
Article4. Barar J, Asadi M, Mortazavi-Tabatabaei SA, Omidi Y. 2009; Ocular drug delivery; impact of In vitro cell culture models. J Ophthalmic Vis Res. 4:238–252.5. Combes RD, Shah AB. 2016; The use of in vivo, ex vivo, in vitro, computational models and volunteer studies in vision research and therapy, and their contribution to the Three Rs. Altern Lab Anim. 44:187–238. DOI: 10.1177/026119291604400302. PMID: 27494623.
Article6. Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. 1996; ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 62:155–169. DOI: 10.1006/exer.1996.0020. PMID: 8698076.
Article7. Hosoya K, Tomi M, Ohtsuki S, Takanaga H, Ueda M, Yanai N, Obinata M, Terasaki T. 2001; Conditionally immor-talized retinal capillary endothelial cell lines (TR-iBRB) expressing differentiated endothelial cell functions derived from a transgenic rat. Exp Eye Res. 72:163–172. DOI: 10.1006/exer.2000.0941. PMID: 11161732.
Article8. Pfeffer BA, Philp NJ. 2014; Cell culture of retinal pigment epithelium: special issue. Exp Eye Res. 126:1–4. DOI: 10.1016/j.exer.2014.07.010. PMID: 25152358.
Article9. Hartnett ME, Lappas A, Darland D, McColm JR, Lovejoy S, D'Amore PA. 2003; Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res. 77:593–599. DOI: 10.1016/S0014-4835(03)00189-1. PMID: 14550401.
Article10. Strauss O. 2005; The retinal pigment epithelium in visual function. Physiol Rev. 85:845–881. DOI: 10.1152/physrev.00021.2004. PMID: 15987797.
Article11. Hansson ML, Albert S, González Somermeyer L, Peco R, Mejía-Ramírez E, Montserrat N, Izpisua Belmonte JC. 2015; Efficient delivery and functional expression of transfected modified mRNA in human embryonic stem cell-derived retinal pigmented epithelial cells. J Biol Chem. 290:5661–5672. DOI: 10.1074/jbc.M114.618835. PMID: 25555917. PMCID: PMC4342478.
Article12. Lakkaraju A, Umapathy A, Tan LX, Daniele L, Philp NJ, Boesze-Battaglia K, Williams DS. 2020; The cell biology of the retinal pigment epithelium. Prog Retin Eye Res. [Epub ahead of print]. DOI: 10.1016/j.preteyeres.2020.100846. PMID: 32105772.
Article13. Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. 2004; Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 6:217–245. DOI: 10.1089/clo.2004.6.217. PMID: 15671670.
Article14. Kashani AH. 2016; Stem cell therapy in nonneovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 57:ORSFm1–9. DOI: 10.1167/iovs.15-17681. PMID: 27116669.
Article15. Nazari H, Zhang L, Zhu D, Chader GJ, Falabella P, Stefanini F, Rowland T, Clegg DO, Kashani AH, Hinton DR, Humayun MS. 2015; Stem cell based therapies for age-related macular degeneration: the promises and the challenges. Prog Retin Eye Res. 48:1–39. DOI: 10.1016/j.preteyeres.2015.06.004. PMID: 26113213.
Article16. De Paiva MRB, Lage NA, Guerra MCA, Mol MPG, Ribeiro MCS, Fulgêncio GO, Gomes DA, Da Costa César I, Fialho SL, Silva-Cunha A. 2019; Toxicity and in vivo release profile of sirolimus from implants into the vitreous of rabbits' eyes. Doc Ophthalmol. 138:3–19. DOI: 10.1007/s10633-018-9664-8. PMID: 30456454.
Article17. Grambergs R, Mondal K, Mandal N. 2019; Inflammatory ocular diseases and sphingolipid signaling. Adv Exp Med Biol. 1159:139–152. DOI: 10.1007/978-3-030-21162-2_8. PMID: 31502203.
Article18. Hassan M, Karkhur S, Bae JH, Halim MS, Ormaechea MS, Onghanseng N, Nguyen NV, Afridi R, Sepah YJ, Do DV, Nguyen QD. 2019; New therapies in development for the management of non-infectious uveitis: a review. Clin Exp Ophthalmol. 47:396–417. DOI: 10.1111/ceo.13511. PMID: 30938012.
Article19. Kaçmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Jabs DA, Levy-Clarke GA, Foster CS. 2010; Cyclosporine for ocular inflammatory diseases. Ophthalmology. 117:576–584. DOI: 10.1016/j.ophtha.2009.08.010. PMID: 20031223. PMCID: PMC2830390.
Article20. Nguyen QD, Merrill PT, Sepah YJ, Ibrahim MA, Banker A, Leonardi A, Chernock M, Mudumba S, Do DV. 2018; Intravitreal sirolimus for the treatment of noninfectious uveitis: evolution through preclinical and clinical studies. Ophthalmology. 125:1984–1993. DOI: 10.1016/j.ophtha.2018.06.015. PMID: 30060978.
Article21. Lee YJ, Kim SW, Seo KY. 2013; Application for tacrolimus ointment in treating refractory inflammatory ocular surface diseases. Am J Ophthalmol. 155:804–813. DOI: 10.1016/j.ajo.2012.12.009. PMID: 23394907.
Article22. Fang CB, Zhou DX, Zhan SX, He Y, Lin Z, Huang C, Li J. 2013; Amelioration of experimental autoimmune uveitis by leflunomide in Lewis rats. PLoS One. 8:e62071. DOI: 10.1371/journal.pone.0062071. PMID: 23626769. PMCID: PMC3633904.
Article23. Robertson SM, Lang LS. 1994; Leflunomide: inhibition of S-antigen induced autoimmune uveitis in Lewis rats. Agents Actions. 42:167–172. DOI: 10.1007/BF01983486. PMID: 7879705.
Article24. Ramos-Casals M, Brito-Zerón P, Bombardieri S, Bootsma H, De Vita S, Dörner T, Fisher BA, Gottenberg JE, Hernandez-Molina G, Kocher A, Kostov B, Kruize AA, Mandl T, Ng WF, Retamozo S, Seror R, Shoenfeld Y, Sisó- Almirall A, Tzioufas AG, Vitali C, Bowman S, Mariette X. 2020; EULAR recommendations for the management of Sjögren's syndrome with topical and systemic therapies. Ann Rheum Dis. 79:3–18. DOI: 10.1136/annrheumdis-2019-216114. PMID: 31672775.
Article25. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. 1998; Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.
Article26. Song MK, Lui GM. 1990; Propagation of fetal human RPE cells: preservation of original culture morphology after serial passage. J Cell Physiol. 143:196–203. DOI: 10.1002/jcp.1041430127. PMID: 2318907.
Article27. Liu Y, Xu HW, Wang L, Li SY, Zhao CJ, Hao J, Li QY, Zhao TT, Wu W, Wang Y, Zhou Q, Qian C, Wang L, Yin ZQ. 2018; Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discov. 4:50. DOI: 10.1038/s41421-018-0053-y. PMID: 30245845. PMCID: PMC6143607.
Article28. Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. 2012; Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 379:713–720. DOI: 10.1016/S0140-6736(12)60028-2. PMID: 22281388.
Article29. Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. 2015; Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 385:509–516. DOI: 10.1016/S0140-6736(14)61376-3.
Article30. Sugino IK, Gullapalli VK, Sun Q, Wang J, Nunes CF, Cheewatrakoolpong N, Johnson AC, Degner BC, Hua J, Liu T, Chen W, Li H, Zarbin MA. 2011; Cell-deposited matrix improves retinal pigment epithelium survival on aged submacular human Bruch's membrane. Invest Ophthalmol Vis Sci. 52:1345–1358. DOI: 10.1167/iovs.10-6112. PMID: 21398292. PMCID: PMC3101675.
Article31. Sugino IK, Sun Q, Wang J, Nunes CF, Cheewatrakoolpong N, Rapista A, Johnson AC, Malcuit C, Klimanskaya I, Lanza R, Zarbin MA. 2011; Comparison of FRPE and human embryonic stem cell-derived RPE behavior on aged human Bruch's membrane. Invest Ophthalmol Vis Sci. 52:4979–4997. DOI: 10.1167/iovs.10-5386. PMID: 21460262. PMCID: PMC3176062.
Article32. Mosmann T. 1983; Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63. DOI: 10.1016/0022-1759(83)90303-4. PMID: 6606682.
Article33. Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. 2006; Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 47:3612–3624. DOI: 10.1167/iovs.05-1622. PMID: 16877436. PMCID: PMC1904392.
Article34. Kokkinaki M, Sahibzada N, Golestaneh N. 2011; Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 29:825–835. DOI: 10.1002/stem.635. PMID: 21480547. PMCID: PMC3322554.
Article35. Fronk AH, Vargis E. 2016; Methods for culturing retinal pigment epithelial cells: a review of current protocols and future recommendations. J Tissue Eng. 7:2041731416650838. DOI: 10.1177/2041731416650838. PMID: 27493715. PMCID: PMC4959307.
Article36. Juuti-Uusitalo K, Vaajasaari H, Ryhänen T, Narkilahti S, Suuronen R, Mannermaa E, Kaarniranta K, Skottman H. 2012; Efflux protein expression in human stem cell-derived retinal pigment epithelial cells. PLoS One. 7:e30089. DOI: 10.1371/journal.pone.0030089. PMID: 22272278. PMCID: PMC3260202.
Article37. Luo Y, Zhuo Y, Fukuhara M, Rizzolo LJ. 2006; Effects of culture conditions on heterogeneity and the apical junctional complex of the ARPE-19 cell line. Invest Ophthalmol Vis Sci. 47:3644–3655. DOI: 10.1167/iovs.06-0166. PMID: 16877439.
Article38. Zarbin MA. 2003; Analysis of retinal pigment epithelium integrin expression and adhesion to aged submacular human Bruch's membrane. Trans Am Ophthalmol Soc. 101:499–520. PMID: 14971591. PMCID: PMC1359002.39. Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA. 2010; The dynamic nature of Bruch's membrane. Prog Retin Eye Res. 29:1–18. DOI: 10.1016/j.preteyeres.2009.08.003. PMID: 19747980.
Article40. Morizur L, Herardot E, Monville C, Ben M'Barek K. 2020; Human pluripotent stem cells: a toolbox to understand and treat retinal degeneration. Mol Cell Neurosci. 107:103523. DOI: 10.1016/j.mcn.2020.103523. PMID: 32634576.
Article41. Mannermaa E, Vellonen KS, Urtti A. 2006; Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 58:1136–1163. DOI: 10.1016/j.addr.2006.07.024. PMID: 17081648.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Brimonidine on Transepithelial Resistance in a Human Retinal Pigment Epithelial Cell Line
- Hydrogen Peroxide-Induced Cell Death in a Human Retinal Pigment Epithelial Cell Line, ARPE-19
- Culture of Retinal Pigment Epithelial Cells on Collagen Membrane
- The Effect of 5-Fluorouraci1 on the Activity of the Retinal Pigment Epithelium in Vitro
- Tunicamycin-induced Endoplasmic Reticulum Stress Upregulates the Expression of Pentraxin 3 in Human Retinal Pigment Epithelial Cells