Metformin Preserves Peripheral Nerve Damage with Comparable Effects to Alpha Lipoic Acid in Streptozotocin/High-Fat Diet Induced Diabetic Rats
- Affiliations
-
- 0Division of Endocrinology and Metabolism, Department of Internal Medicine, Jeonbuk National University Medical School, Research Institute of Clinical Medicine of Jeonbuk National University-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, Korea.
- KMID: 2513048
- DOI: http://doi.org/10.4093/dmj.2019.0190
Abstract
Background Metformin is widely marketed medication for the treatment of diabetes, but its pharmacological effect on diabetic peripheral neuropathy remains unclear. In this study, the effect of metformin on peripheral nerves in diabetic rats was investigated using diverse neuronal parameters of nerve fibers.
Methods Rats were assigned to one of four groups (
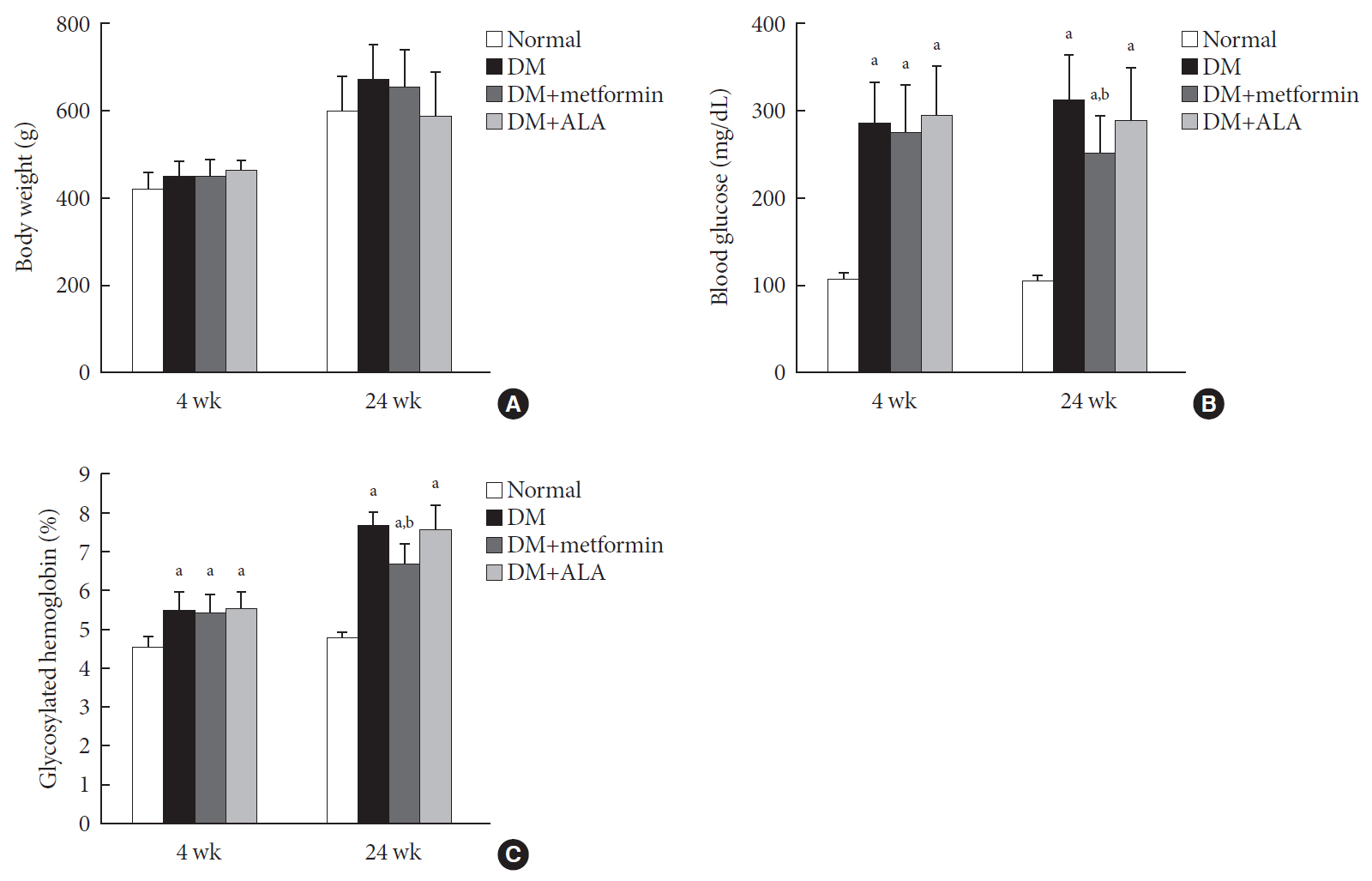
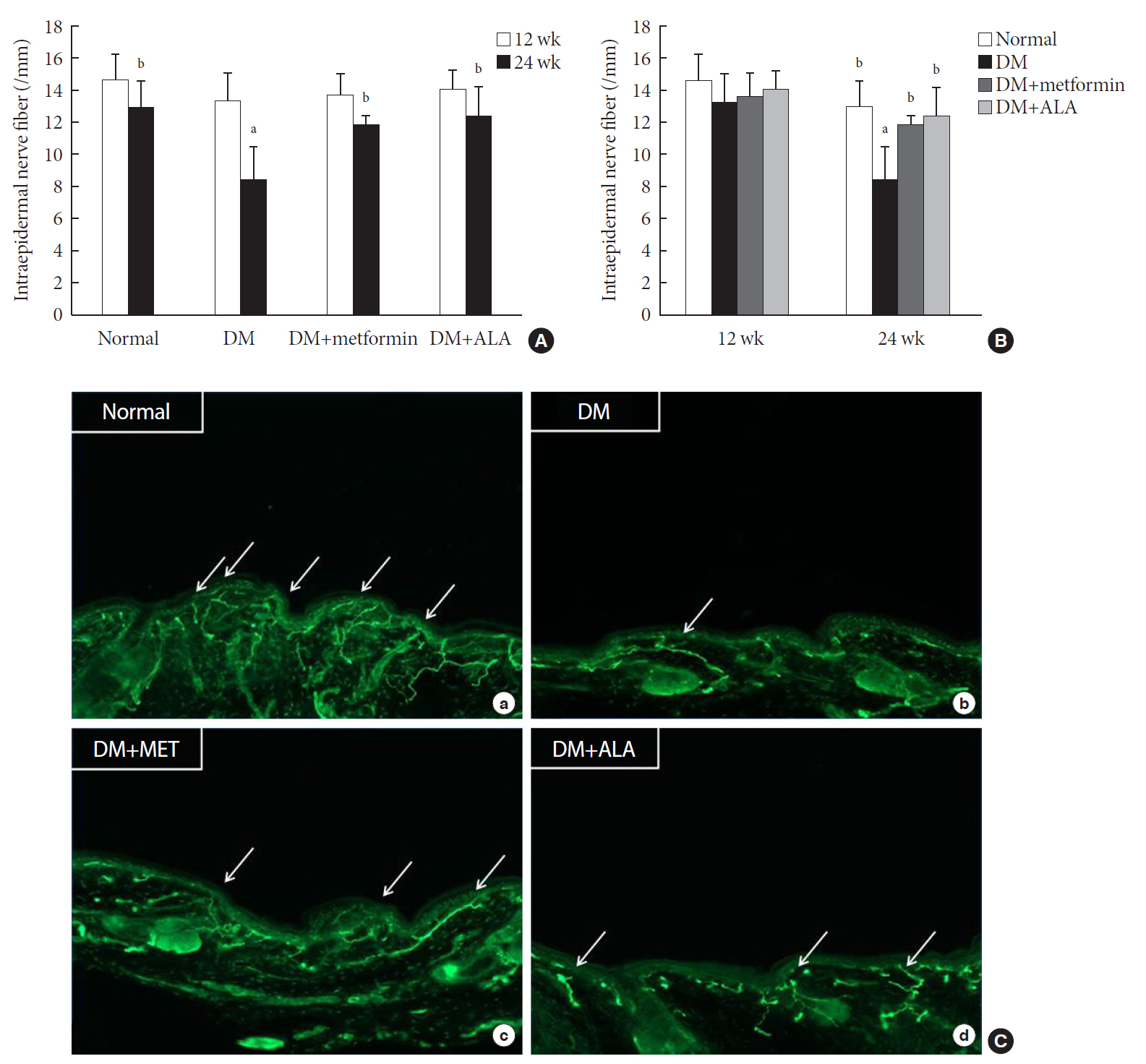
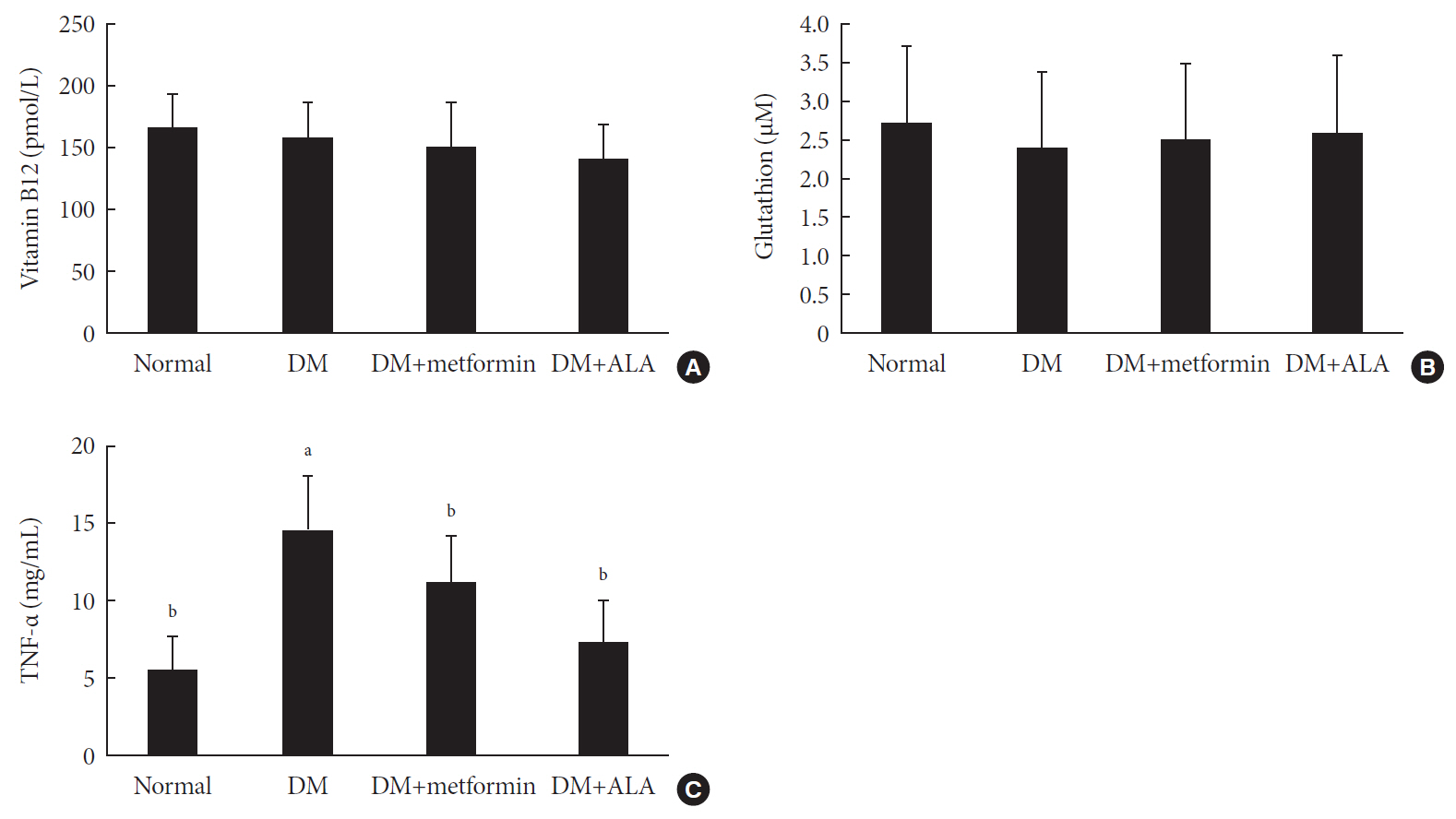
n =7 to 10 per group): normal, diabetes mellitus (DM), DM+metformin (100 mg/kg), and DM+alpha lipoic acid (ALA, 100 mg/kg). DM was induced by streptozotocin/high-fat diet (STZ/HFD). After 12 weeks, the sensory thresholds to mechanical and heat stimuli were assessed. Repeated sensory tests, immunofluorescence microscopic comparison of peripheral nerves, and biochemical blood analysis were performed after 24 weeks.Results Both DM+metformin and DM+ALA groups showed similar trends to diverse sensory tests at 24 weeks compared to DM group although the degree of change were different according to the stimulated senses. There was no significant difference in the comparison of the intraepidermal nerve fiber density (IENFD) of peripheral nerves between the DM+metformin and DM+ALA groups (11.83±0.07 fibers/mm vs. 12.37±1.82 fibers/mm, respectively). Both groups showed preserved IENFD significantly compared with DM group (8.46±1.98 fibers/mm,
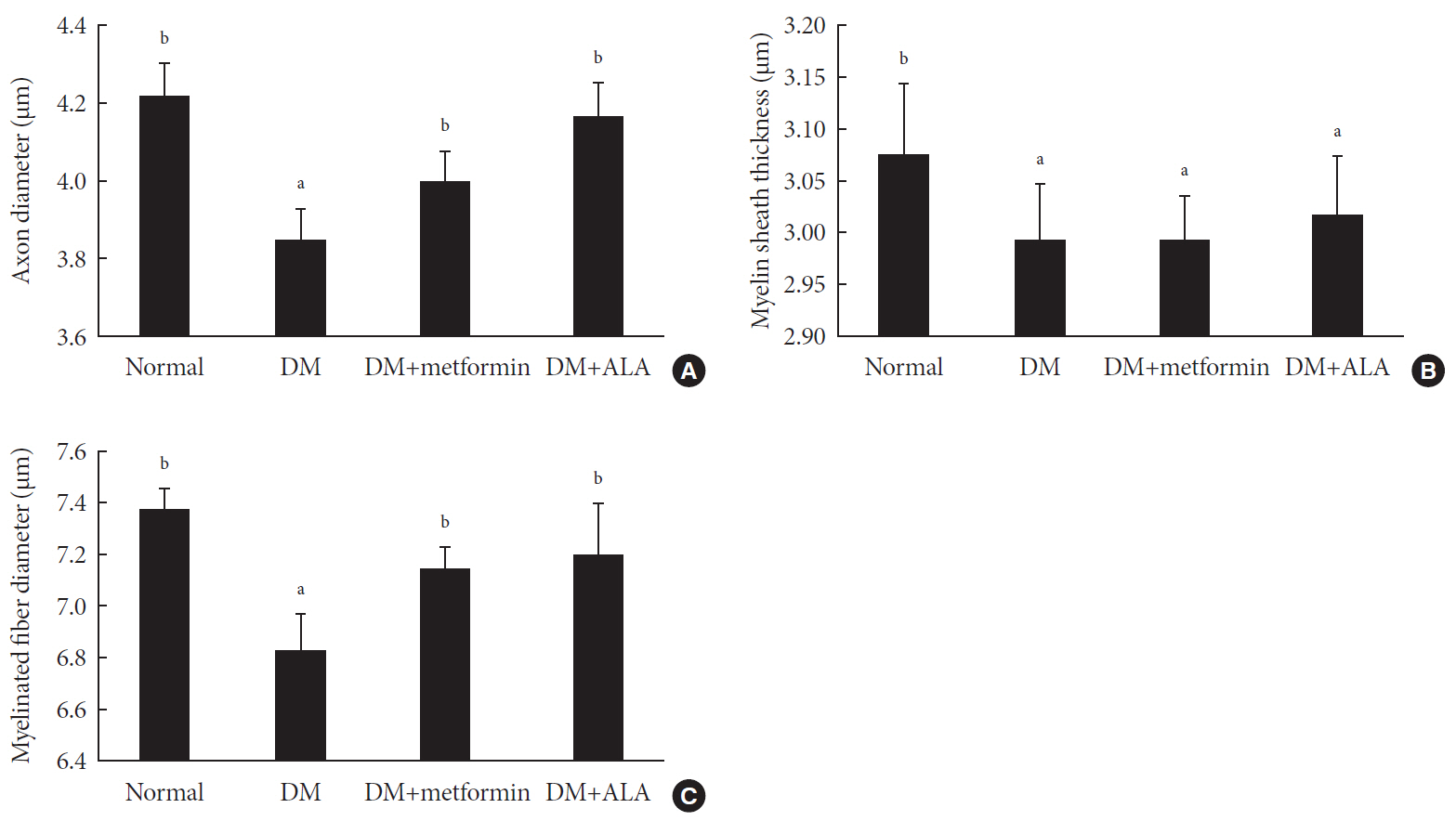
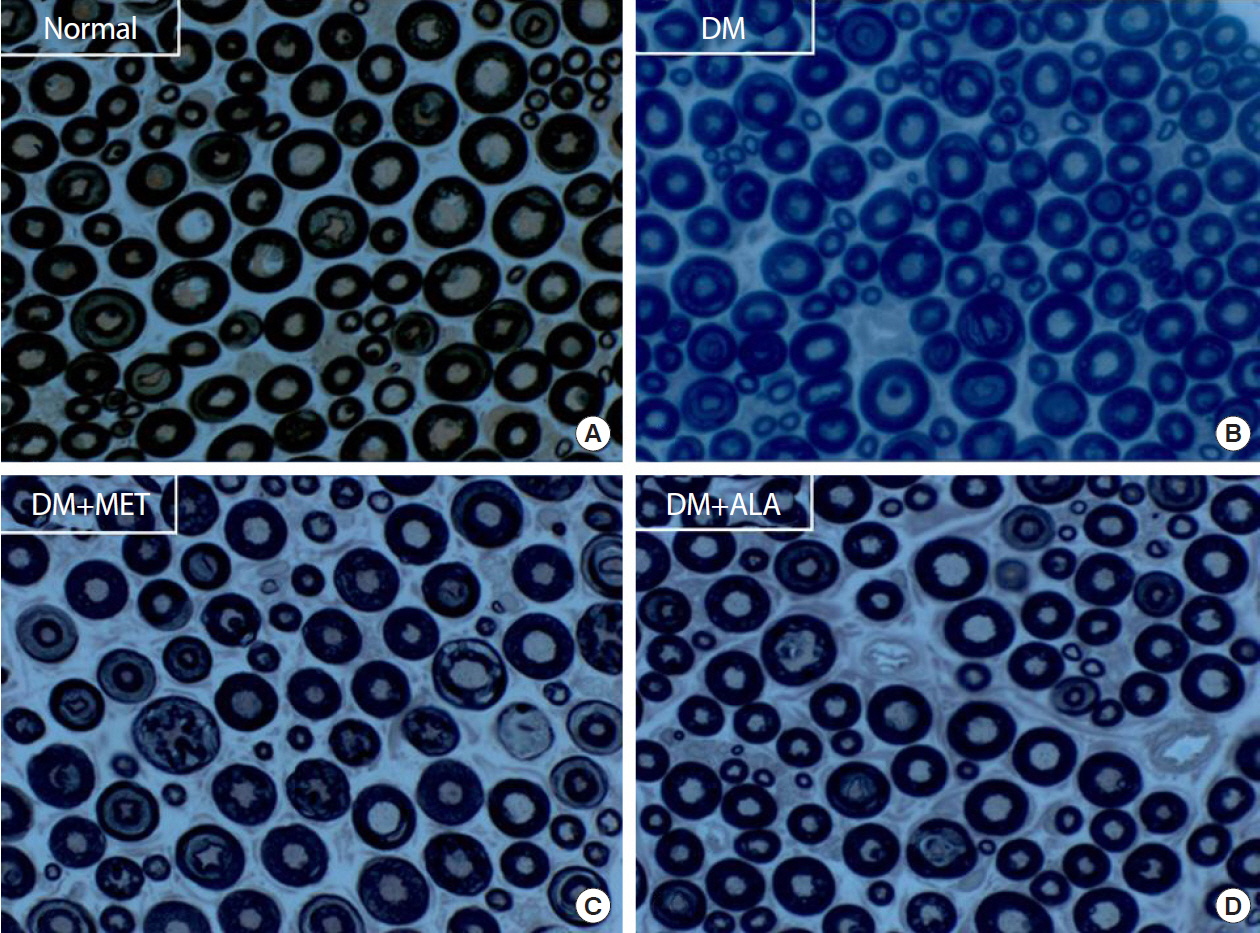
P <0.05). Sciatic nerve morphology of the experimental animals showed a similar trend to the IENFD, with respect to axonal diameter, myelin sheath thickness, and myelinated fiber diameter.Conclusion Metformin has beneficial pharmacological effects on the preservation of peripheral nerves in diabetic rats and its effects are comparable to those of ALA.
Figure
Cited by 3 articles
-
The Anti-Diabetic Drug Metformin from the Neuropathy Perspective
Jong Chul Won
Diabetes Metab J. 2020;44(6):840-841. doi: 10.4093/dmj.2020.0252.Metformin Preserves Peripheral Nerve Damage with Comparable Effects to Alpha Lipoic Acid in Streptozotocin/High-Fat Diet Induced Diabetic Rats (
Diabetes Metab J 2020;44:842-53)
Bo Kyung Koo
Diabetes Metab J. 2021;45(1):125-126. doi: 10.4093/dmj.2020.0280.Metformin Preserves Peripheral Nerve Damage with Comparable Effects to Alpha Lipoic Acid in Streptozotocin/High-Fat Diet Induced Diabetic Rats (
Diabetes Metab J 2020;44:842-53)
Sun Hee Kim, Tae Sun Park, Heung Yong Jin
Diabetes Metab J. 2021;45(1):127-128. doi: 10.4093/dmj.2020.0289.
Reference
-
1. Tesfaye S. Advances in the management of diabetic peripheral neuropathy. Curr Opin Support Palliat Care. 2009; 3:136–143.
Article2. Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000; 43:957–973.
Article3. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010; 33:2285–2293.
Article4. Griggs RB, Donahue RR, Adkins BG, Anderson KL, Thibault O, Taylor BK. Pioglitazone inhibits the development of hyperalgesia and sensitization of spinal nociresponsive neurons in type 2 diabetes. J Pain. 2016; 17:359–373.
Article5. Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002; 300:958–966.
Article6. Jin HY, Liu WJ, Park JH, Baek HS, Park TS. Effect of dipeptidyl peptidase-IV (DPP-IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats. Arch Med Res. 2009; 40:536–544.
Article7. Maeda T, Kiguchi N, Kobayashi Y, Ozaki M, Kishioka S. Pioglitazone attenuates tactile allodynia and thermal hyperalgesia in mice subjected to peripheral nerve injury. J Pharmacol Sci. 2008; 108:341–347.
Article8. Wiggin TD, Kretzler M, Pennathur S, Sullivan KA, Brosius FC, Feldman EL. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology. 2008; 149:4928–4937.
Article9. Biswas SC, Buteau J, Greene LA. Glucagon-like peptide-1 (GLP-1) diminishes neuronal degeneration and death caused by NGF deprivation by suppressing Bim induction. Neurochem Res. 2008; 33:1845–1851.
Article10. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019; 42:S90–S102.11. Tomkin GH, Hadden DR, Weaver JA, Montgomery DA. Vitamin-B12 status of patients on long-term metformin therapy. Br Med J. 1971; 2:685–687.
Article12. de Jager J, Kooy A, Lehert P, Wulffele MG, van der Kolk J, Bets D, Verburg J, Donker AJ, Stehouwer CD. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010; 340:c2181.
Article13. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017; 40:136–154.
Article14. Ahmed MA, Muntingh G, Rheeder P. Vitamin B12 deficiency in metformin-treated type-2 diabetes patients, prevalence and association with peripheral neuropathy. BMC Pharmacol Toxicol. 2016; 17:44.
Article15. Elhadd T, Ponirakis G, Dabbous Z, Siddique M, Chinnaiyan S, Malik RA. Metformin use is not associated with B(12) deficiency or neuropathy in patients with type 2 diabetes mellitus in Qatar. Front Endocrinol (Lausanne). 2018; 9:248.
Article16. Zhou W, Kavelaars A, Heijnen CJ. Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PLoS One. 2016; 11:e0151890.
Article17. Taylor A, Westveld AH, Szkudlinska M, Guruguri P, Annabi E, Patwardhan A, Price TJ, Yassine HN. The use of metformin is associated with decreased lumbar radiculopathy pain. J Pain Res. 2013; 6:755–763.18. Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. 2014; 126:3–22.
Article19. Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996; 19:257–267.
Article20. Apfel SC. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999; 41:27–34.
Article21. Bonhof GJ, Herder C, Strom A, Papanas N, Roden M, Ziegler D. Emerging biomarkers, tools, and treatments for diabetic polyneuropathy. Endocr Rev. 2019; 40:153–192.22. Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, Bray GA, Schade DS, Temprosa MG, White NH, Crandall JP. Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab. 2016; 101:1754–1761.
Article23. Gupta K, Jain A, Rohatgi A. An observational study of vitamin b12 levels and peripheral neuropathy profile in patients of diabetes mellitus on metformin therapy. Diabetes Metab Syndr. 2018; 12:51–58.
Article24. Ma J, Yu H, Liu J, Chen Y, Wang Q, Xiang L. Metformin attenuates hyperalgesia and allodynia in rats with painful diabetic neuropathy induced by streptozotocin. Eur J Pharmacol. 2015; 764:599–606.
Article25. El-Mir MY, Detaille D, R-Villanueva G, Delgado-Esteban M, Guigas B, Attia S, Fontaine E, Almeida A, Leverve X. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci. 2008; 34:77–87.
Article26. Melemedjian OK, Asiedu MN, Tillu DV, Sanoja R, Yan J, Lark A, Khoutorsky A, Johnson J, Peebles KA, Lepow T, Sonenberg N, Dussor G, Price TJ. Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain. Mol Pain. 2011; 7:70.
Article27. Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci. 2012; 13:11.
Article28. Patil SP, Jain PD, Ghumatkar PJ, Tambe R, Sathaye S. Neuroprotective effect of metformin in MPTP-induced Parkinson's disease in mice. Neuroscience. 2014; 277:747–754.
Article29. Roy Chowdhury SK, Smith DR, Saleh A, Schapansky J, Marquez A, Gomes S, Akude E, Morrow D, Calcutt NA, Fernyhough P. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012; 135:1751–1766.
Article30. Beiswenger KK, Calcutt NA, Mizisin AP. Dissociation of thermal hypoalgesia and epidermal denervation in streptozotocin-diabetic mice. Neurosci Lett. 2008; 442:267–272.
Article31. Goncalves NP, Vaegter CB, Andersen H, Ostergaard L, Calcutt NA, Jensen TS. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol. 2017; 13:135–147.
Article32. Lee KA, Lee NY, Park TS, Jin HY. Comparison of peripheral nerve protection between insulin-based glucose control and alpha lipoic acid (ALA) in the streptozotocin (STZ)-induced diabetic rat. Endocrine. 2018; 61:58–67.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metformin Preserves Peripheral Nerve Damage with Comparable Effects to Alpha Lipoic Acid in Streptozotocin/High-Fat Diet Induced Diabetic Rats (Diabetes Metab J 2020;44:842-53)
- Metformin Preserves Peripheral Nerve Damage with Comparable Effects to Alpha Lipoic Acid in Streptozotocin/High-Fat Diet Induced Diabetic Rats (Diabetes Metab J 2020;44:842-53)
- alpha-Lipoic acid reduced weight gain and improved the lipid profile in rats fed with high fat diet
- The Effect of Alpha-Lipoic Acid on the Protection of Epidermal Nerve Fibers and Microcapillaries in the Streptozotocin-Induced Diabetic Rats
- Neurotrophic Effect of alpha-Lipoic Acid on Diabetic Neuropathy