Diabetes Metab J.
2020 Dec;44(6):802-818. 10.4093/dmj.2020.0258.
Comprehensive Review of Current and Upcoming Anti-Obesity Drugs
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2513044
- DOI: http://doi.org/10.4093/dmj.2020.0258
Abstract
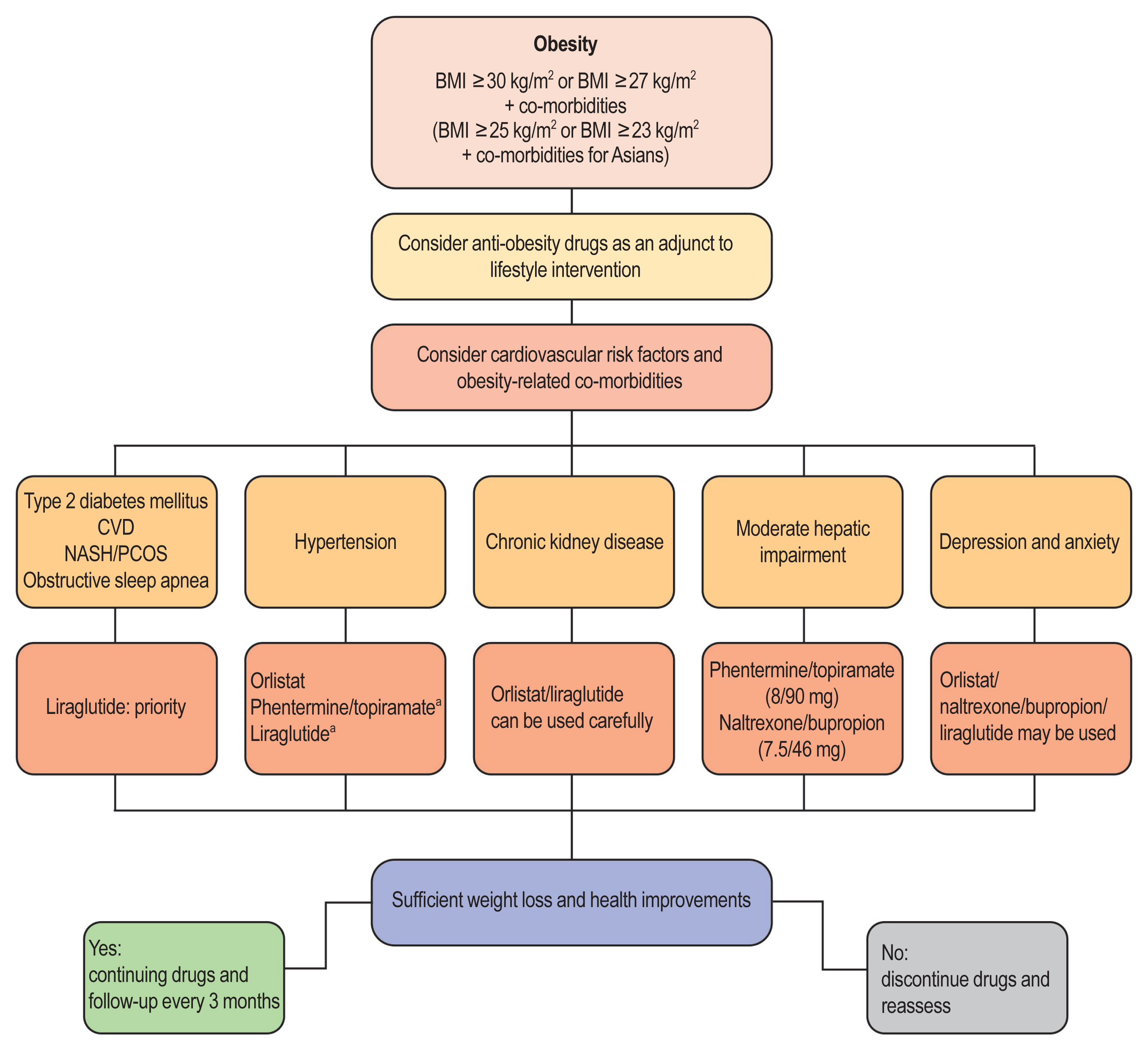
- Obesity is among the leading causes of morbidity and mortality worldwide and its prevalence continues to increase globally. Because obesity is a chronic, complex, and heterogeneous disease influenced by genetic, developmental, biological, and environmental factors, it is necessary to approach obesity with an integrated and comprehensive treatment strategy. As it is difficult to achieve and sustain successful long-term weight loss in most patients with obesity through lifestyle modifications (e.g., diet, exercise, and behavioral therapy), pharmacological approaches to the treatment of obesity should be considered as an adjunct therapy. Currently, four drugs (orlistat, naltrexone extended-release [ER]/bupropion ER, phentermine/topiramate controlled-release, and liraglutide) can be used long-term (>12 weeks) to promote weight loss by suppressing appetite or decreasing fat absorption. Pharmacotherapy for obesity should be conducted according to a proper assessment of the clinical evidence and customized to individual patients considering the characteristics of each drug and comorbidities associated with obesity. In this review, we discuss the mechanisms of action, efficacy, and safety of these available long-term anti-obesity drugs and introduce other potential agents under investigation. Furthermore, we discuss the need for research on personalized obesity medicine.
Keyword
Figure
Cited by 1 articles
-
Management Strategies for Obese Diabetes
Mi-Sook Kim, Seung-Hwan Lee
J Korean Diabetes. 2024;25(3):165-171. doi: 10.4093/jkd.2024.25.3.165.
Reference
-
1. Haslam DW, James WP. Obesity. Lancet. 2005; 366:1197–209.
Article2. O’Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008; 57:2905–10.
Article3. WHO Consultation on Obesity. Obesity: preventing and managing the global epidemic. Geneva: World Health Organization;2000.4. Korean Society for the Study of Obesity, National Health Insurance Service. 2019 Obesity fact sheet. Seoul: Korean Society for the Study of Obesity;2019.5. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017; 390:2627–42.6. Swinburn BA, Kraak VI, Allender S, Atkins VJ, Baker PI, Bogard JR, Brinsden H, Calvillo A, De Schutter O, Devarajan R, Ezzati M, Friel S, Goenka S, Hammond RA, Hastings G, Hawkes C, Herrero M, Hovmand PS, Howden M, Jaacks LM, Kapetanaki AB, Kasman M, Kuhnlein HV, Kumanyika SK, Larijani B, Lobstein T, Long MW, Matsudo VKR, Mills SDH, Morgan G, Morshed A, Nece PM, Pan A, Patterson DW, Sacks G, Shekar M, Simmons GL, Smit W, Tootee A, Vandevijvere S, Waterlander WE, Wolfenden L, Dietz WH. The global syndemic of obesity, undernutrition, and climate change: the Lancet Commission report. Lancet. 2019; 393:791–846.
Article7. Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD. Endocrine Society. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015; 100:342–62.
Article8. World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia;2000.9. Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, Kushner RF, Daniels SR, Wadden TA, Tsai AG, Hu FB, Jakicic JM, Ryan DH, Wolfe BM, Inge TH. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018; 39:79–132.
Article10. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, Toplak H. Obesity Management Task Force of the European Association for the Study of Obesity. European guidelines for obesity management in adults. Obes Facts. 2015; 8:402–24.
Article11. Sharretts J, Galescu O, Gomatam S, Andraca-Carrera E, Hampp C, Yanoff L. Cancer risk associated with Lorcaserin: the FDA’s Review of the CAMELLIA-TIMI 61 Trial. N Engl J Med. 2020; 383:1000–2.12. U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for industry developing products for weight management. Available from: https://www.fda.gov/media/71252/download (cited 2020 Dec 8).13. Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018; 14:12–24.
Article14. Hvizdos KM, Markham A. Orlistat: a review of its use in the management of obesity. Drugs. 1999; 58:743–60.15. Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000; 20:270–9.
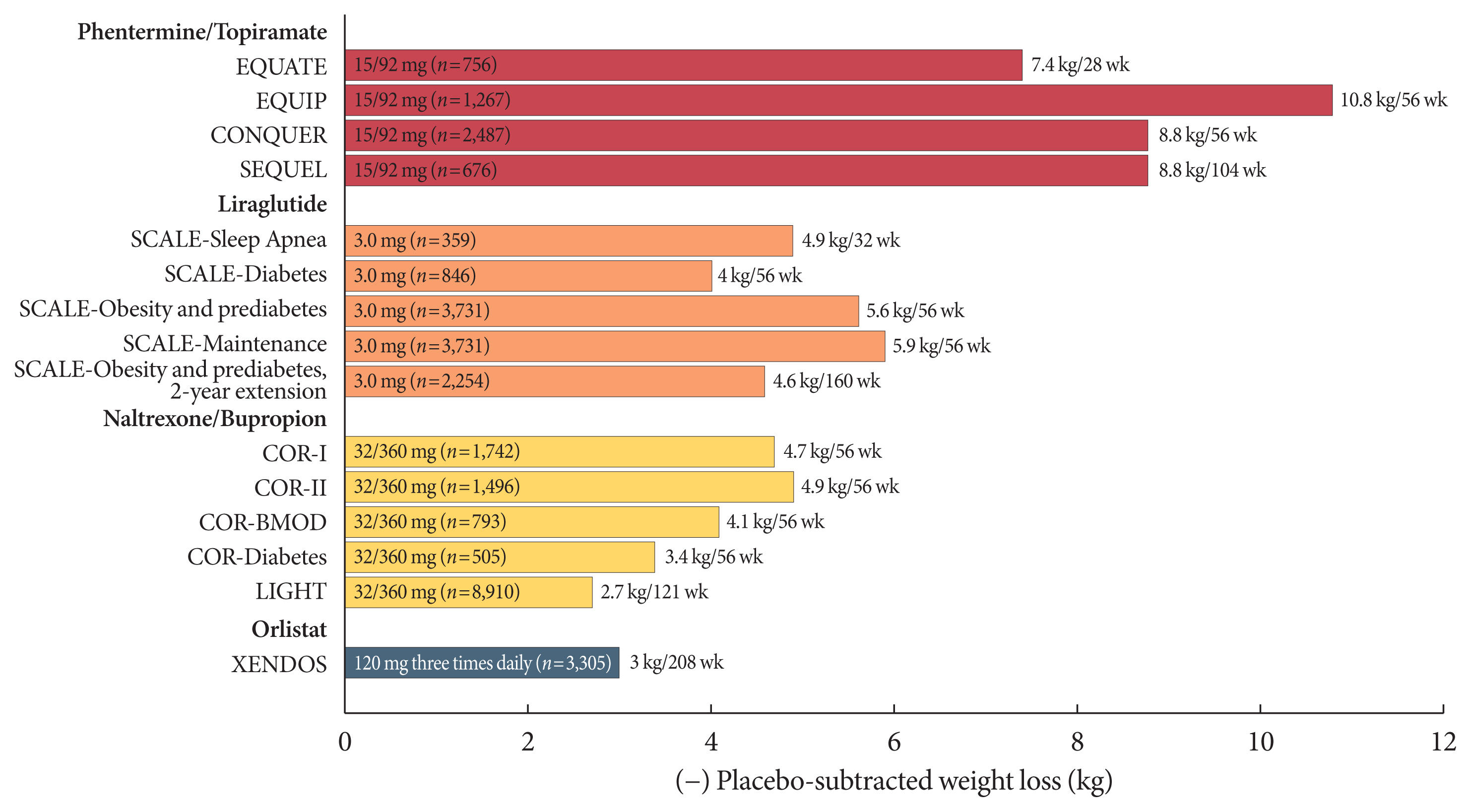
Article16. Drew BS, Dixon AF, Dixon JB. Obesity management: update on orlistat. Vasc Health Risk Manag. 2007; 3:817–21.17. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004; 27:155–61.
Article18. Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007; 335:1194–9.
Article19. Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med. 2000; 9:160–7.
Article20. Genentech. Xenical (Orlistat) package insert. Available from: https://www.gene.com(cited 2020 Dec 8).21. Ballinger A, Peikin SR. Orlistat: its current status as an anti-obesity drug. Eur J Pharmacol. 2002; 440:109–17.
Article22. Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005; 293:2873–83.
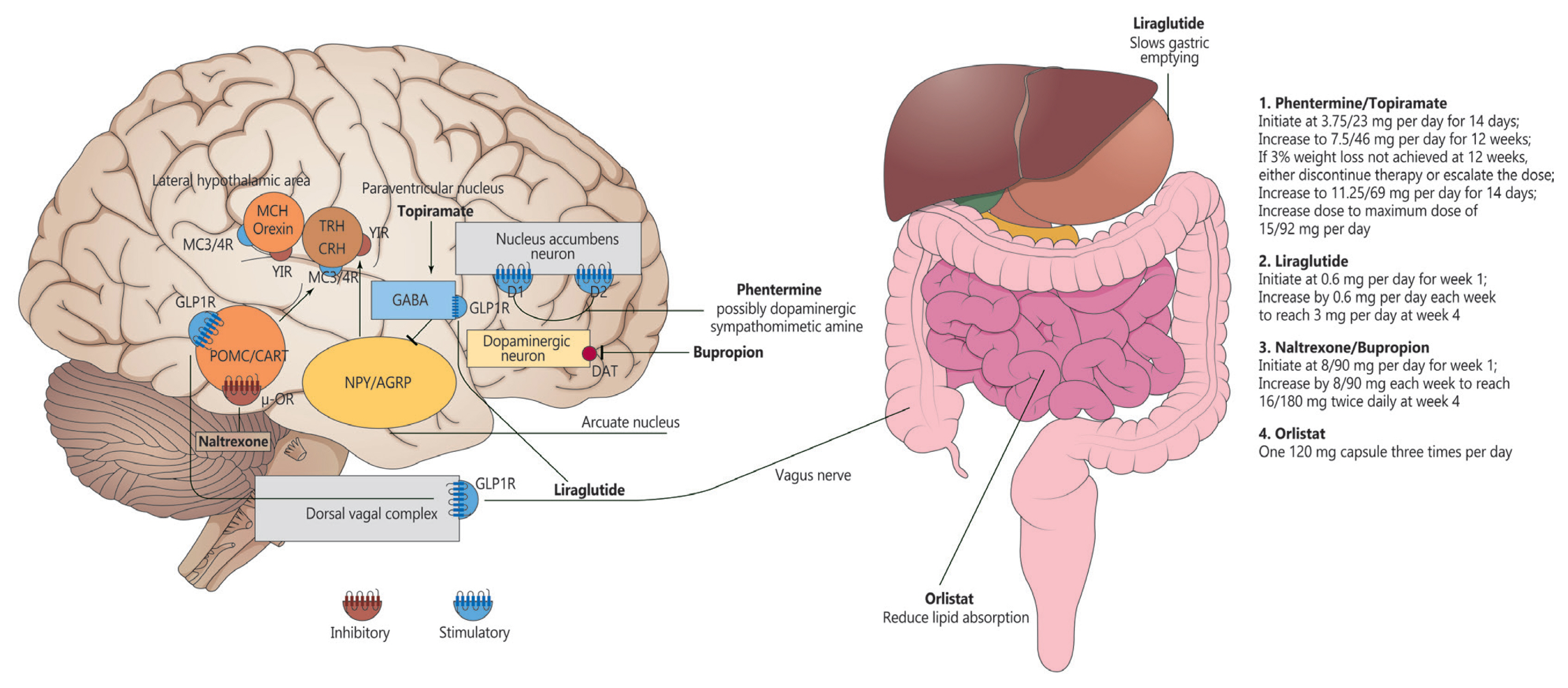
Article23. Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001; 357:354–7.
Article24. Caixas A, Albert L, Capel I, Rigla M. Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date. Drug Des Devel Ther. 2014; 8:1419–27.
Article25. Koch M, Varela L, Kim JG, Kim JD, Hernandez-Nuno F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buck K, Dietrich MO, Gao XB, Diano S, Horvath TL. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015; 519:45–50.
Article26. Dutia R, Meece K, Dighe S, Kim AJ, Wardlaw SL. β-Endorphin antagonizes the effects of α-MSH on food intake and body weight. Endocrinology. 2012; 153:4246–55.
Article27. Greig SL, Keating GM. Naltrexone ER/bupropion ER: a review in obesity management. Drugs. 2015; 75:1269–80.
Article28. Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E. COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010; 376:595–605.
Article29. Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, Kim D, Dunayevich E. COR-II Study Group. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013; 21:935–43.
Article30. Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM, Perri MG, Pi-Sunyer FX, Rock CL, Erickson JS, Maier HN, Kim DD, Dunayevich E. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011; 19:110–20.
Article31. Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, Klassen P, Fujioka K. COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013; 36:4022–9.32. Smith SR, Fujioka K, Gupta AK, Billes SK, Burns C, Kim D, Dunayevich E, Greenway FL. Combination therapy with naltrexone and bupropion for obesity reduces total and visceral adiposity. Diabetes Obes Metab. 2013; 15:863–6.
Article33. U.S. Food Drug Administration. Contrave package insert. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200063s000lbl.pdf(cited 2020 Dec 8).34. Nissen SE, Wolski KE, Prcela L, Wadden T, Buse JB, Bakris G, Perez A, Smith SR. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016; 315:990–1004.35. Antel J, Hebebrand J. Weight-reducing side effects of the antiepileptic agents topiramate and zonisamide. Handb Exp Pharmacol. 2012; 433–66.
Article36. Pilitsi E, Farr OM, Polyzos SA, Perakakis N, Nolen-Doerr E, Papathanasiou AE, Mantzoros CS. Pharmacotherapy of obesity: available medications and drugs under investigation. Metabolism. 2019; 92:170–92.
Article37. Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011; 377:1341–52.
Article38. Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012; 20:330–42.
Article39. Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, Singh S. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016; 315:2424–34.40. Mines D, Tennis P, Curkendall SM, Li DK, Peterson C, Andrews EB, Calingaert B, Chen H, Deshpande G, Esposito DB, Everage N, Holick CN, Meyer NM, Nkhoma ET, Quinn S, Rothman KJ, Chan KA. Topiramate use in pregnancy and the birth prevalence of oral clefts. Pharmacoepidemiol Drug Saf. 2014; 23:1017–25.
Article41. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP. SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015; 373:11–22.
Article42. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, Andreasen AH, Jensen CB, DeFronzo RA. NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015; 314:687–99.43. Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, Aronne L. NN8022-1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond). 2013; 37:1443–51.
Article44. Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, Claudius B, Jensen CB, Mignot E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. Int J Obes (Lond). 2016; 40:1310–9.45. Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012; 95:297–308.
Article46. Qsymia. Qsymia (phentermine and topiramate extended-release) package insert. Available from: https://www.qsymia.com(cited 2020 Dec 8).47. Colman E, Golden J, Roberts M, Egan A, Weaver J, Rosebraugh C. The FDA’s assessment of two drugs for chronic weight management. N Engl J Med. 2012; 367:1577–9.
Article48. Torekov SS, Madsbad S, Holst JJ. Obesity: an indication for GLP-1 treatment? Obesity pathophysiology and GLP-1 treatment potential. Obes Rev. 2011; 12:593–601.49. Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011; 7:507–16.
Article50. Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002; 18:7–14.
Article51. Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, Hansen G, Grove KL, Pyke C, Raun K, Schaffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014; 124:4473–88.
Article52. le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, Ortiz RV, Wilding JPH, Skjoth TV, Manning LS, Pi-Sunyer X. SCALE Obesity Prediabetes NN8022-1839 Study Group. 3 Years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017; 389:1399–409.53. Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rossner S, Savolainen MJ, Van Gaal L. NN8022-1807 Investigators. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012; 36:843–54.
Article54. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hubscher SG, Newsome PN. LEAN trial team. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016; 387:679–90.
Article55. Nylander M, Frossing S, Clausen HV, Kistorp C, Faber J, Skouby SO. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: a randomized clinical trial. Reprod Biomed Online. 2017; 35:121–7.
Article56. Miras AD, Perez-Pevida B, Aldhwayan M, Kamocka A, Mc-Glone ER, Al-Najim W, Chahal H, Batterham RL, McGowan B, Khan O, Greener V, Ahmed AR, Petrie A, Scholtz S, Bloom SR, Tan TM. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019; 7:549–59.
Article57. Chalmer T, Almdal TP, Vilsboll T, Knop FK. Adverse drug reactions associated with the use of liraglutide in patients with type 2 diabetes: focus on pancreatitis and pancreas cancer. Expert Opin Drug Saf. 2015; 14:171–80.58. Monami M, Nreu B, Scatena A, Cresci B, Andreozzi F, Sesti G, Mannucci E. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab. 2017; 19:1233–41.59. U.S. Food Drug Administration. Saxenda (package insert). Liraglutide (rDNA) injection. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206321Orig1s000lbl.pdf (cited 2020 Dec 8).60. Gallo M. Thyroid safety in patients treated with liraglutide. J Endocrinol Invest. 2013; 36:140–5.
Article61. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like peptide-1 receptor agonists activate rodent thyroid Ccells causing calcitonin release and C-cell proliferation. Endocrinology. 2010; 151:1473–86.
Article62. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–22.
Article63. Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018; 6:237–48.
Article64. Ten Kulve JS, Veltman DJ, van Bloemendaal L, Barkhof F, Drent ML, Diamant M, IJzerman RG. Liraglutide reduces CNS activation in response to visual food cues only after short-term treatment in patients with type 2 diabetes. Diabetes Care. 2016; 39:214–21.
Article65. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014; 38:784–93.
Article66. Wang GJ, Tomasi D, Volkow ND, Wang R, Telang F, Caparelli EC, Dunayevich E. Effect of combined naltrexone and bupropion therapy on the brain’s reactivity to food cues. Int J Obes (Lond). 2014; 38:682–8.
Article67. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016; 22(Suppl 3):1–203.
Article68. Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep. 2012; 35:1529–39.
Article69. Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin Pharmacol Ther. 2014; 95:53–66.
Article70. Jeong JK, Kim JG, Lee BJ. Participation of the central melanocortin system in metabolic regulation and energy homeostasis. Cell Mol Life Sci. 2014; 71:3799–809.
Article71. Clement K, Biebermann H, Farooqi IS, Van der Ploeg L, Wolters B, Poitou C, Puder L, Fiedorek F, Gottesdiener K, Kleinau G, Heyder N, Scheerer P, Blume-Peytavi U, Jahnke I, Sharma S, Mokrosinski J, Wiegand S, Muller A, Weib K, Mai K, Spranger J, Gruters A, Blankenstein O, Krude H, Kuhnen P. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med. 2018; 24:551–5.
Article72. Kuhnen P, Clement K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, Mai K, Blume-Peytavi U, Gruters A, Krude H. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016; 375:240–6.
Article73. Haws R, Brady S, Davis E, Fletty K, Yuan G, Gordon G, Stewart M, Yanovski J. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes Metab. 2020; 22:2133–40.74. Astrup A, Madsbad S, Breum L, Jensen TJ, Kroustrup JP, Larsen TM. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet. 2008; 372:1906–13.
Article75. Sjodin A, Gasteyger C, Nielsen AL, Raben A, Mikkelsen JD, Jensen JK, Meier D, Astrup A. The effect of the triple monoamine reuptake inhibitor tesofensine on energy metabolism and appetite in overweight and moderately obese men. Int J Obes (Lond). 2010; 34:1634–43.76. Saniona. Tesofensine monotherapy for treatment of obesity. Available from: https://saniona.com(cited 2020 Dec 8).77. Roberts CA, Christiansen P, Halford JCG. Tailoring pharmacotherapy to specific eating behaviours in obesity: can recommendations for personalized therapy be made from the current data? Acta Diabetol. 2017; 54:715–25.