Schemes and Performance Evaluation Criteria of Korean Association of External Quality Assessment (KEQAS) for Improving Laboratory Testing
- Affiliations
-
- 1Department of Laboratory Medicine, Inje University, Ilsan Paik Hospital, Goyang, Korea
- 2Department of Laboratory Medicine, Seoul National University Bundang Hospital and College of Medicine, Seongnam, Korea
- 3Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Laboratory Medicine and Genetics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- 5Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- KMID: 2512671
- DOI: http://doi.org/10.3343/alm.2021.41.2.230
Abstract
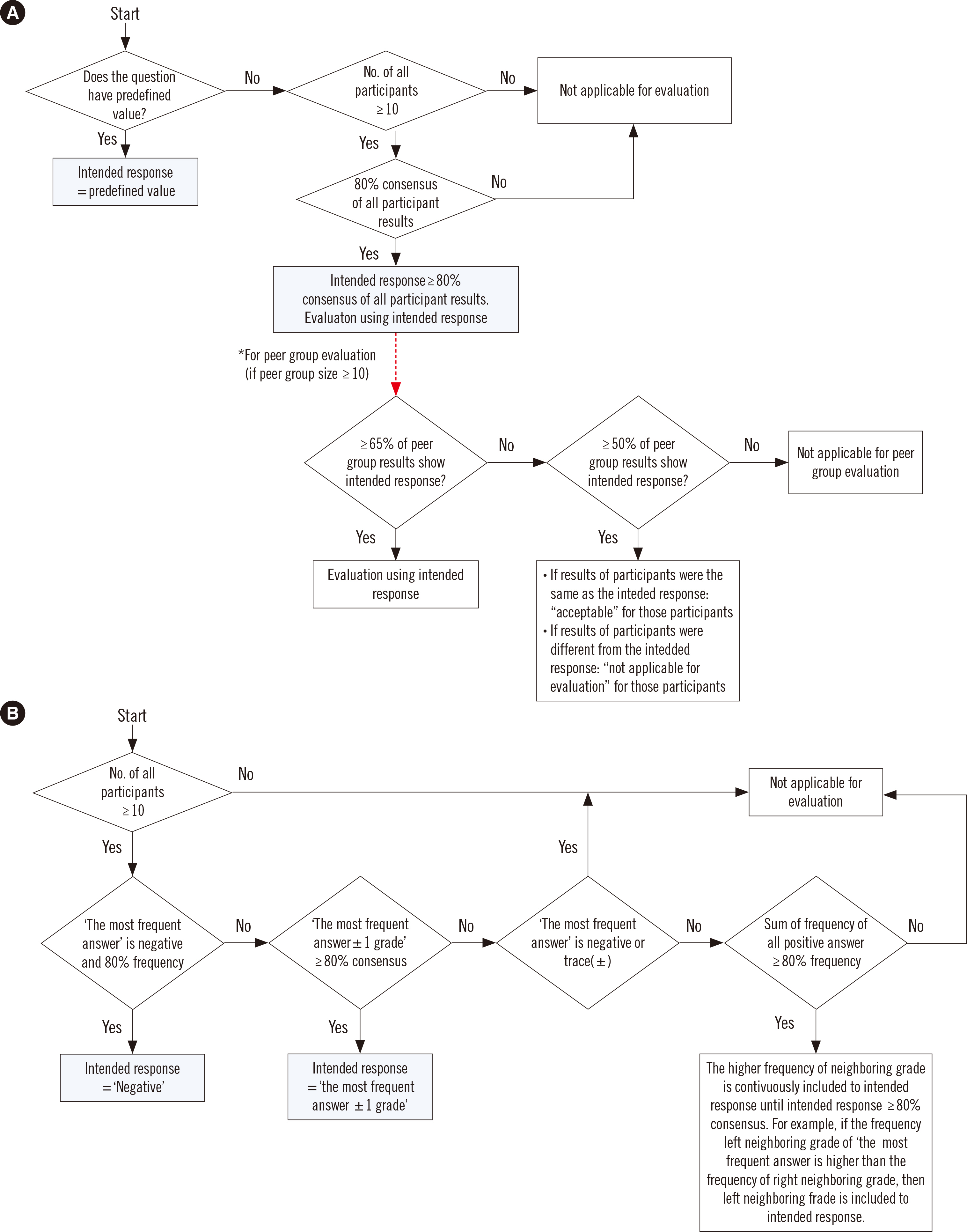
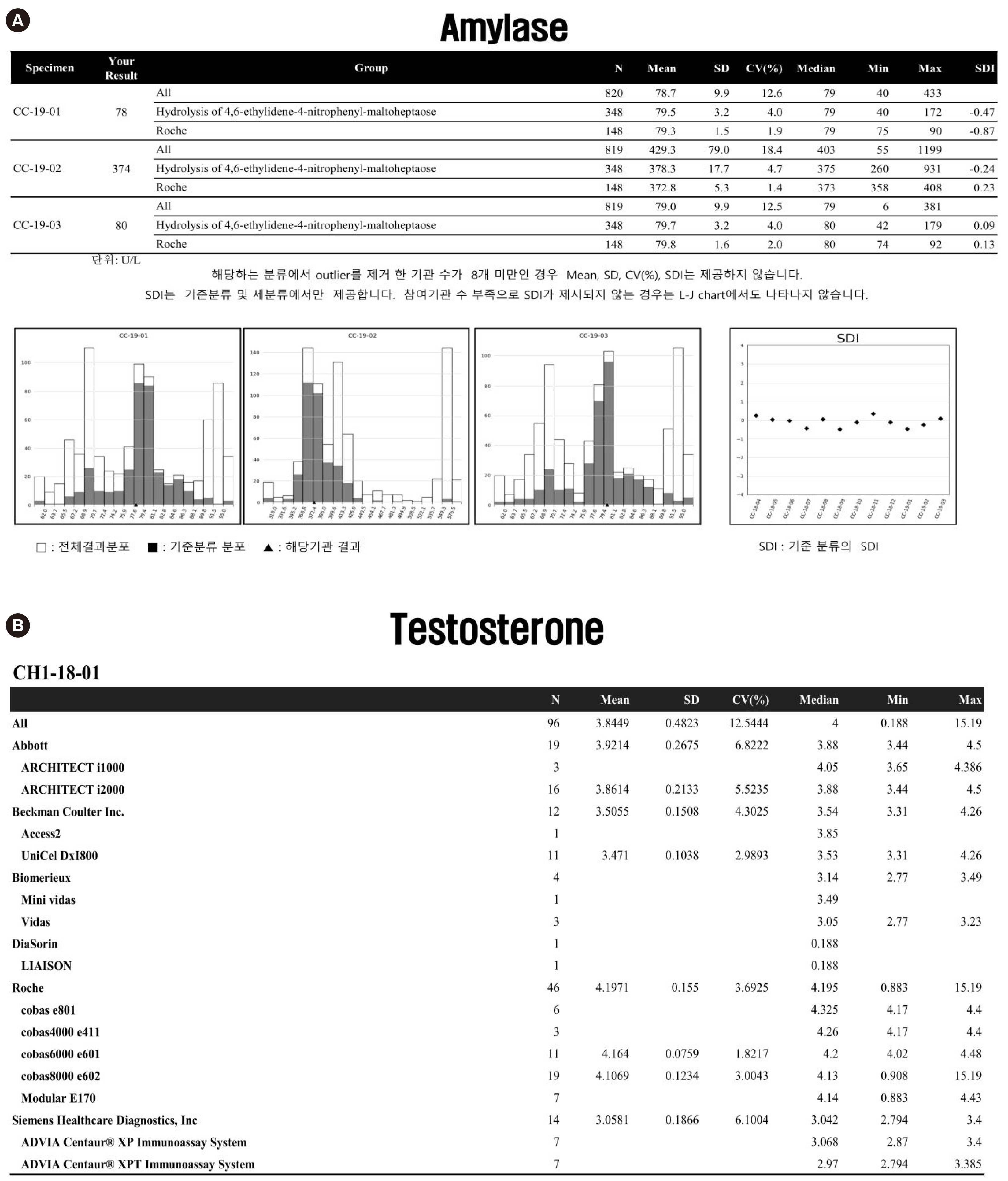
- External quality assessment (EQA) is important for evaluating clinical laboratories and enhancing their testing quality. EQA schemes are variable; thus, it is crucial that the EQA organizers share their experiences to continuously improve the EQA scheme. The Korean Association of External Quality Assessment Service (KEQAS) has been the leading, authorized EQA institute for the standardization and quality management of laboratory testing in Korean medical institutions since 1976. The EQA scheme underwent a major change in 2016, and the number of EQA programs increased significantly since then. The key changes implemented in EQA scheme include a fully computerized assessment to accelerate feedback and unification of the testing and reporting methods. We provide an overview of the EQA schemes and performance evaluation criteria of the KEQAS and suggest directions for achieving the global harmonization of EQA.
Keyword
Figure
Cited by 8 articles
-
Standardization Status of Total Cholesterol Concentration Measurement: Analysis of Korean External Quality Assessment Data
Young Ahn Yoon, Yong-Wha Lee, Sollip Kim, Kyunghoon Lee, Hyung-Doo Park, Sail Chun, Won-Ki Min
Ann Lab Med. 2021;41(4):366-371. doi: 10.3343/alm.2021.41.4.366.Periodic Comparability Verification and Within-Laboratory Harmonization of Clinical Chemistry Laboratory Results at a Large Healthcare Center With Multiple Instruments
Youngwon Nam, Joon Hee Lee, Sung Min Kim, Sun-Hee Jun, Sang Hoon Song, Kyunghoon Lee, Junghan Song
Ann Lab Med. 2022;42(2):150-159. doi: 10.3343/alm.2022.42.2.150.Age Group-specific Reference Intervals for the Elecsys Anti-Müllerian Hormone Assay in Healthy Korean Women: a Nationwide Population-based Study
Misuk Ji, Kwang-Rae Kim, Hyun-Ki Kim, Woochang Lee, Yeo-Min Yun, Sail Chun, Won-Ki Min
Ann Lab Med. 2022;42(6):621-629. doi: 10.3343/alm.2022.42.6.621.Age-specific Reference Intervals for Anti-Müllerian Hormone
Haeil Park
Ann Lab Med. 2022;42(6):617-618. doi: 10.3343/alm.2022.42.6.617.Proposed Model for Evaluating Real-world Laboratory Results for Big Data Research
Sollip Kim, Eun-Jung Cho, Tae-Dong Jeong, Hyung-Doo Park, Yeo-Min Yun, Kyunghoon Lee, Yong-Wha Lee, Sail Chun, Won-Ki Min
Ann Lab Med. 2023;43(1):104-107. doi: 10.3343/alm.2023.43.1.104.Laboratory Data Quality Evaluation in the Big Data Era
Sollip Kim
Ann Lab Med. 2023;43(5):399-400. doi: 10.3343/alm.2023.43.5.399.A New Strategy for Evaluating the Quality of Laboratory Results for Big Data Research: Using External Quality Assessment Survey Data (2010–2020)
Eun-Jung Cho, Tae-Dong Jeong, Sollip Kim, Hyung-Doo Park, Yeo-Min Yun, Sail Chun, Won-Ki Min
Ann Lab Med. 2023;43(5):425-433. doi: 10.3343/alm.2023.43.5.425.Quality Status of the Preanalytical Phase of Clinical Laboratories in Korea
Yuna Choi, Kyunghoon Lee, Hyun-Jung Choi, Soo Young Moon, Jinsook Lim, Sollip Kim
Lab Med Online. 2024;14(2):90-99. doi: 10.47429/lmo.2024.14.2.90.
Reference
-
1. Jang MA, Yoon YA, Song J, Kim JH, Min WK, Lee JS, et al. 2017; Effect of accreditation on accuracy of diagnostic in medical laboratories. Ann Lab Med. 37:213–22. DOI: 10.3343/alm.2017.37.3.213. PMID: 28224767. PMCID: PMC5339093.2. Lee W, Ryoo N, Suh HS, Jeon CH. 2019; Improvement in external quality assessment results for qualitative fecal immunochemical tests in Korea after feedback to manufacturers. Ann Lab Med. 39:584–6. DOI: 10.3343/alm.2019.39.6.584. PMID: 31240889. PMCID: PMC6660328.
Article3. World Health Organization. 2016. WHO manual for organizing a national external quality assessment programme for health laboratories and other testing sites. WHO Press;Geneva, Switzerland:4. Ceriotti F, Cobbaert C. 2018; Harmonization of External Quality Assessment Schemes and their role-clinical chemistry and beyond. Clin Chem Lab Med. 56:1587–90. DOI: 10.1515/cclm-2018-0265. PMID: 29715181.5. Haliassos A. 2018; Inter-laboratory comparisons and EQA in the Mediterranean area. EJIFCC. 29:253–8.6. Peterson JC, Hill RH, Black RS, Winkelman J, Tholen D. 2008. Review of proficiency testing services for clinical laboratories in the United States-final report of a Technical Working Group. Battelle Memorial Institute; Atlanta, GA:7. Kim S, Yun YM, Kim H, Um TH, Chang J, Jeong H, et al. 2020; The new diagnosis-related group reimbursement system and laboratory test quality in Korea: analysis of external quality assessment results. Healthcare (Basel). 8:127. DOI: 10.3390/healthcare8020127. PMID: 32392746. PMCID: PMC7349770.
Article8. Korean Association of External Quality Assessment Service. https://keqas.org/. Updated on Sep 2020.9. Kim H, Kim S, Yun YM, Um TH, Chang J, Lee KS, et al. 2020; Status of quality control for laboratory tests of medical institutions in Korea: analysis of 10 years of data on external quality assessment participation. Healthcare (Basel). 8:75. DOI: 10.3390/healthcare8020075. PMID: 32230819. PMCID: PMC7349217.
Article10. Miller WG, Jones GR, Horowitz GL, Weykamp C. 2011; Proficiency testing/external quality assessment: current challenges and future directions. Clin Chem. 57:1670–80. DOI: 10.1373/clinchem.2011.168641. PMID: 21965556.
Article11. Jeong TD, Lee HA, Lee K, Yun YM. 2019; Accuracy-based proficiency testing of creatinine measurement: 7 years' experience in Korea. J Lab Med Qual Assur. 41:13–23. DOI: 10.15263/jlmqa.2019.41.1.13.
Article12. Krleza JL, Celap I, Tanaskovic JV. 2017; External Quality Assessment in Croatia: problems, challenges, and specific circumstances. Biochem Med (Zagreb). 27:86–92. DOI: 10.11613/BM.2017.011. PMID: 28392730. PMCID: PMC5382851.13. Jones GRD, Albarede S, Kesseler D, MacKenzie F, Mammen J, Pedersen M, et al. 2017; Analytical performance specifications for external quality assessment-definitions and descriptions. Clin Chem Lab Med. 55:949–55. DOI: 10.1515/cclm-2017-0151. PMID: 28593915.
Article14. CLSI. 2016. Using proficiency testing and alternative assessment to improve medical laboratory quality. CLSI QMS24. 3rd ed. Clinical and Laboratory Standards Institute;Wayne, PA:15. Coucke W, Soumali MR. 2017; Demystifying EQA statistics and reports. Biochem Med (Zagreb). 27:37–48. DOI: 10.11613/BM.2017.006. PMID: 28392725. PMCID: PMC5382862.
Article16. Lippi G, Chance JJ, Church S, Dazzi P, Fontana R, Giavarina D, et al. 2011; Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med. 49:1113–26. DOI: 10.1515/CCLM.2011.600. PMID: 21517699.
Article17. Plebani M, Sciacovelli L, Aita A, Padoan A, Chiozza ML. 2014; Quality indicators to detect pre-analytical errors in laboratory testing. Clin Chim Acta. 432:44–8. DOI: 10.1016/j.cca.2013.07.033. PMID: 24012653.
Article18. Duan M, Kang F, Zhao H, Wang W, Du Y, He F, et al. 2019; Analysis and evaluation of the external quality assessment results of quality indicators in laboratory medicine all over China from 2015 to 2018. Clin Chem Lab Med. 57:812–21. DOI: 10.1515/cclm-2018-0983. PMID: 30511924.
Article19. Badrick T, Gay S, McCaughey EJ, Georgiou A. 2017; External Quality Assessment beyond the analytical phase: an Australian perspective. Biochem Med (Zagreb). 27:73–80. DOI: 10.11613/BM.2017.009. PMID: 28392728. PMCID: PMC5382854.
Article20. Bachner P. 2014; Anniversary of Q-Probes and Q-Tracks quality assurance programs. Arch Pathol Lab Med. 138:1139–40. DOI: 10.5858/arpa.2014-0244-ED. PMID: 25171695.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- External Quality Assessment and Clinical Laboratory Guidelines for Serum Protein and Immunofixation Electrophoresis in Korea
- Report of the Korean Association of External Quality Assessment Service on Clinical Mycobacteriology (2017–2018)
- Analysis of Unacceptable Results in Proficiency Testing for General Transfusion Medicine Tests
- Report of the Korean Association of External Quality Assessment Service on Accuracy-Based Glucose Testing (2022)
- Analyses of the Proficiency Testing Program and the Performance of Qualitative Reagents for Rheumatoid Factor