J Stroke.
2021 Jan;23(1):12-36. 10.5853/jos.2020.03349.
Development and Testing of Thrombolytics in Stroke
- Affiliations
-
- 1International Centre for Clinical Research, St. Anne’s Hospital, Brno, Czech Republic
- 2Loschmidt Laboratories, Department of Experimental Biology and RECETOX, Faculty of Science, Masaryk University, Brno, Czech Republic
- 3Molecular Imaging and Neurovascular Research Laboratory, Department of Neurology, Dongguk University College of Medicine, Goyang, Korea
- 4Department of Neurology, St. Anne’s Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic
- 5Department of Neurology, Dongguk University Ilsan Hospital, Goyang, Korea
- KMID: 2512352
- DOI: http://doi.org/10.5853/jos.2020.03349
Abstract
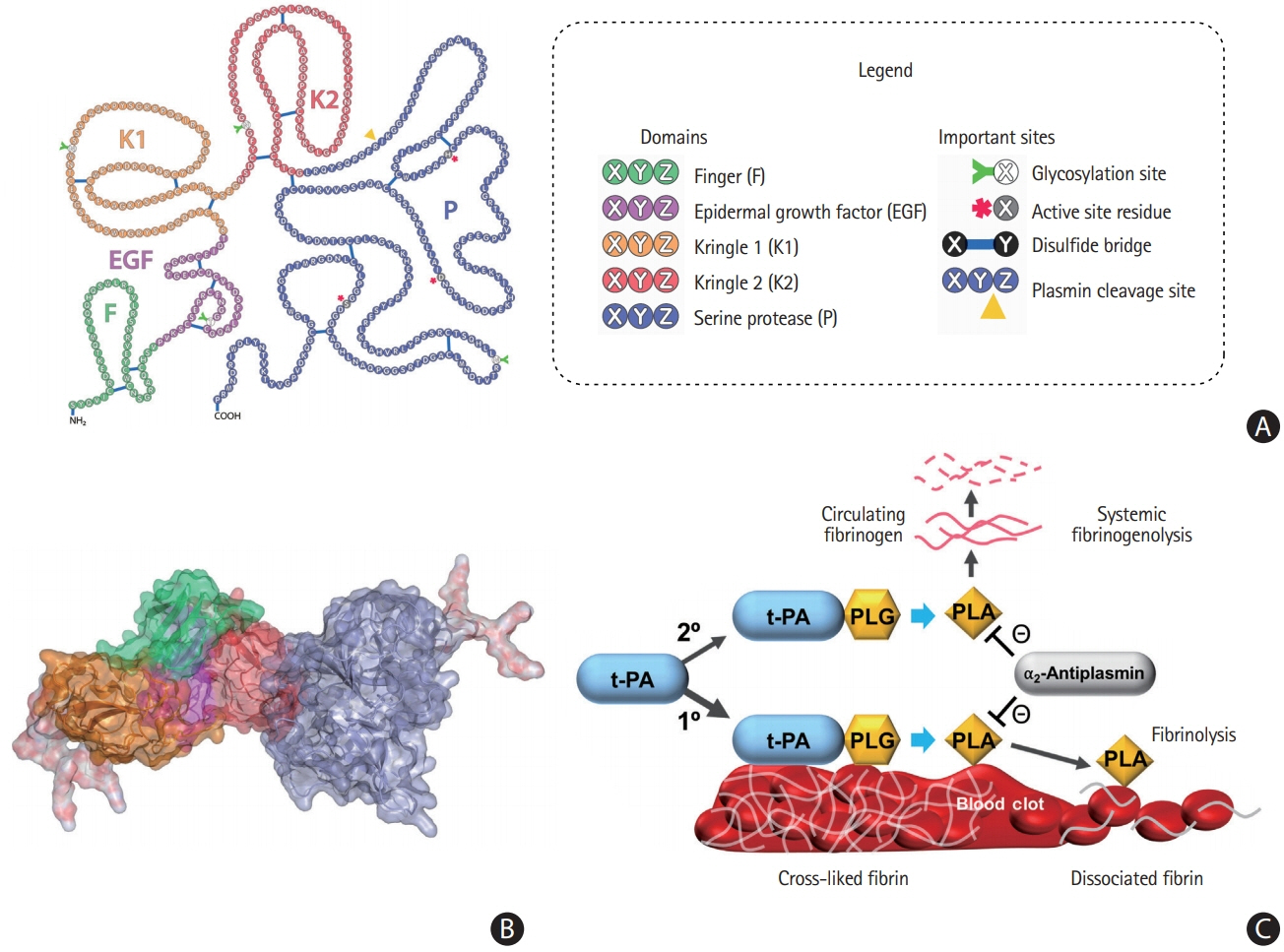
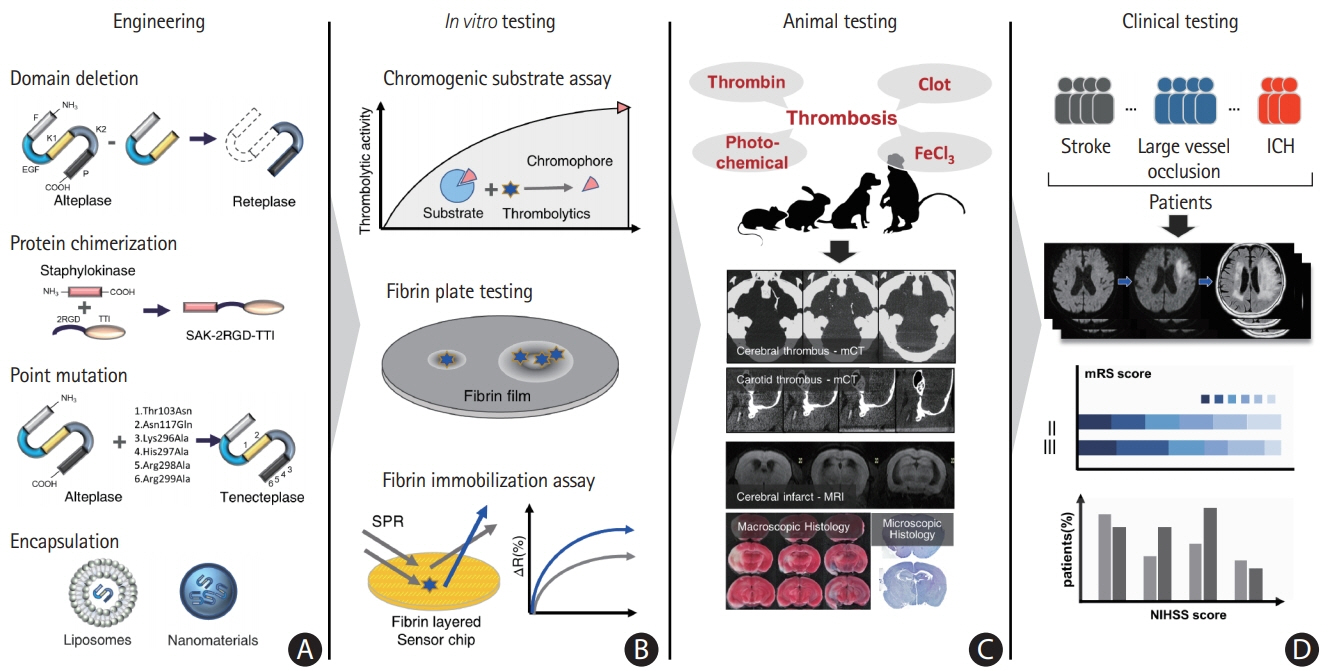
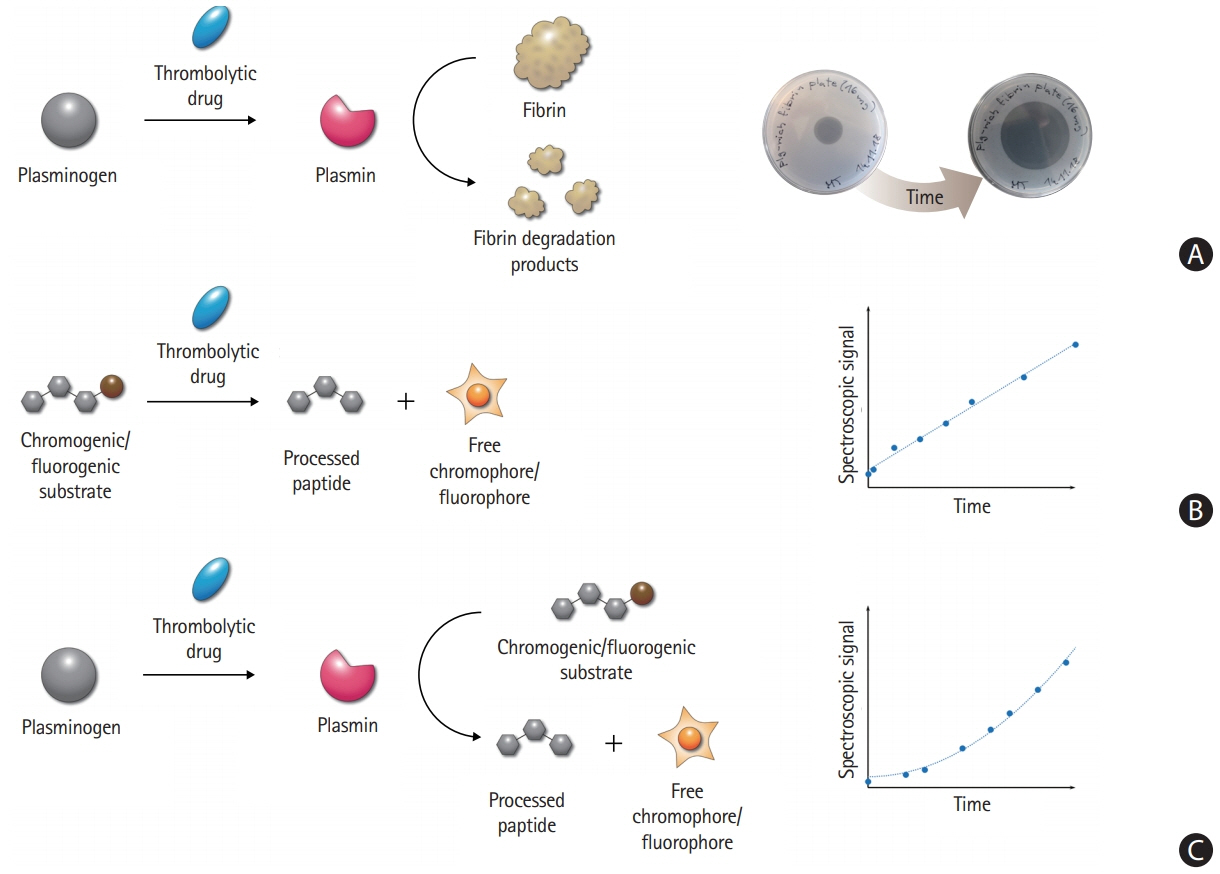
- Despite recent advances in recanalization therapy, mechanical thrombectomy will never be a treatment for every ischemic stroke because access to mechanical thrombectomy is still limited in many countries. Moreover, many ischemic strokes are caused by occlusion of cerebral arteries that cannot be reached by intra-arterial catheters. Reperfusion using thrombolytic agents will therefore remain an important therapy for hyperacute ischemic stroke. However, thrombolytic drugs have shown limited efficacy and notable hemorrhagic complication rates, leaving room for improvement. A comprehensive understanding of basic and clinical research pipelines as well as the current status of thrombolytic therapy will help facilitate the development of new thrombolytics. Compared with alteplase, an ideal thrombolytic agent is expected to provide faster reperfusion in more patients; prevent re-occlusions; have higher fibrin specificity for selective activation of clot-bound plasminogen to decrease bleeding complications; be retained in the blood for a longer time to minimize dosage and allow administration as a single bolus; be more resistant to inhibitors; and be less antigenic for repetitive usage. Here, we review the currently available thrombolytics, strategies for the development of new clot-dissolving substances, and the assessment of thrombolytic efficacies in vitro and in vivo.
Figure
Reference
-
References
1. GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019; 18:439–458.2. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008; 359:1317–1329.
Article3. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015; 372:2296–2306.
Article4. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030.5. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378:708–718.
Article6. Aguiar de Sousa D, von Martial R, Abilleira S, Gattringer T, Kobayashi A, Gallofré M, et al. Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J. 2019; 4:13–28.
Article7. Kim JS. tPA helpers in the treatment of acute ischemic stroke: are they ready for clinical use? J Stroke. 2019; 21:160–174.
Article8. Rijken DC, Wijngaards G, Zaal-de Jong M, Welbergen J. Purification and partial characterization of plasminogen activator from human uterine tissue. Biochim Biophys Acta. 1979; 580:140–153.
Article9. Rijken DC, Hoylaerts M, Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem. 1982; 257:2920–2925.
Article10. Angles-Cano E, Balaton A, Le Bonniec B, Genot E, Elion J, Sultan Y. Production of monoclonal antibodies to the high fibrin-affinity, tissue-type plasminogen activator of human plasma: demonstration of its endothelial origin by immunolocalization. Blood. 1985; 66:913–920.11. Correa F, Gauberti M, Parcq J, Macrez R, Hommet Y, Obiang P, et al. Tissue plasminogen activator prevents white matter damage following stroke. J Exp Med. 2011; 208:1229–1242.
Article12. Louessard M, Lacroix A, Martineau M, Mondielli G, Montagne A, Lesept F, et al. Tissue plasminogen activator expression is restricted to subsets of excitatory pyramidal glutamatergic neurons. Mol Neurobiol. 2016; 53:5000–5012.
Article13. Ny T, Elgh F, Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984; 81:5355–5359.
Article14. Lijnen HR, Collen D. Strategies for the improvement of thrombolytic agents. Thromb Haemost. 1991; 66:88–110.
Article15. Gething MJ, Adler B, Boose JA, Gerard RD, Madison EL, Mc-Gookey D, et al. Variants of human tissue-type plasminogen activator that lack specific structural domains of the heavy chain. EMBO J. 1988; 7:2731–2740.
Article16. Collen D, Lijnen HR. Tissue-type plasminogen activator: a historical perspective and personal account. J Thromb Haemost. 2004; 2:541–546.
Article17. Lucore CL, Fry ET, Nachowiak DA, Sobel BE. Biochemical determinants of clearance of tissue-type plasminogen activator from the circulation. Circulation. 1988; 77:906–914.
Article18. Bakhit C, Lewis D, Billings R, Malfroy B. Cellular catabolism of recombinant tissue-type plasminogen activator. Identification and characterization of a novel high affinity uptake system on rat hepatocytes. J Biol Chem. 1987; 262:8716–8720.
Article19. Pennica D, Holmes WE, Kohr WJ, Harkins RN, Vehar GA, Ward CA, et al. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983; 301:214–221.
Article20. Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998; 21:813–825.
Article21. Lee SH, Ko HM, Kwon KJ, Lee J, Han SH, Han DW, et al. tPA regulates neurite outgrowth by phosphorylation of LRP5/6 in neural progenitor cells. Mol Neurobiol. 2014; 49:199–215.
Article22. Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001; 7:59–64.
Article23. Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, et al. Cleavage of proBDNF by tPA/plasmin is essential for longterm hippocampal plasticity. Science. 2004; 306:487–491.
Article24. Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003; 6:168–174.
Article25. Seeds NW, Basham ME, Haffke SP. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc Natl Acad Sci U S A. 1999; 96:14118–14123.
Article26. Su EJ, Fredriksson L, Schielke GP, Eriksson U, Lawrence DA. Tissue plasminogen activator-mediated PDGF signaling and neurovascular coupling in stroke. J Thromb Haemost. 2009; 7 Suppl 1:155–158.
Article27. Sila CA, Furlan AJ. Therapy for acute ischemic stroke: the door opens: interpreting the NINDS rt-PA stroke study. Cleve Clin J Med. 1996; 63:77–79.
Article28. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014; 384:1929–1935.
Article29. Suzuki S, Saito M, Suzuki N, Kato H, Nagaoka N, Yoshitake S, et al. Thrombolytic properties of a novel modified human tissue-type plasminogen activator (E6010): a bolus injection of E6010 has equivalent potency of lysing young and aged canine coronary thrombi. J Cardiovasc Pharmacol. 1991; 17:738–746.30. den Heijer P, Vermeer F, Ambrosioni E, Sadowski Z, López-Sendón JL, von Essen R, et al. Evaluation of a weight-adjusted single-bolus plasminogen activator in patients with myocardial infarction: a double-blind, randomized angiographic trial of lanoteplase versus alteplase. Circulation. 1998; 98:2117–2125.31. Ishikawa A, Ohata T, Imamura K, Iwasaki M, Sakai T, Matsuzawa T, et al. Single and repeated intravenous toxicity studies of pamiteplase (genetical recombination) in rats and monkeys. J Toxicol Sci. 1997; 22:117–133.
Article32. Agnelli G, Pascucci C, Nenci GG, Mele A, Bürgi R, Heim J. Thrombolytic and haemorrhagic effects of bolus doses of tissue-type plasminogen activator and a hybrid plasminogen activator with prolonged plasma half-life (K2tu-PA: CGP 42935). Thromb Haemost. 1993; 70:294–300.
Article33. Narita M, Bu G, Herz J, Schwartz AL. Two receptor systems are involved in the plasma clearance of tissue-type plasminogen activator (t-PA) in vivo. J Clin Invest. 1995; 96:1164–1168.
Article34. Kuiper J, Van’t Hof A, Otter M, Biessen EA, Rijken DC, van Berkel TJ. Interaction of mutants of tissue-type plasminogen activator with liver cells: effect of domain deletions. Biochem J. 1996; 313:775–780.
Article35. Rathore YS, Rehan M, Pandey K, Sahni G. First structural model of full-length human tissue-plasminogen activator: a SAXS data-based modeling study. J Phys Chem B. 2012; 116:496–502.
Article36. Mican J, Toul M, Bednar D, Damborsky J. Structural biology and protein engineering of thrombolytics. Comput Struct Biotechnol J. 2019; 17:917–938.
Article37. Keyt BA, Paoni NF, Refino CJ, Berleau L, Nguyen H, Chow A, et al. A faster-acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci U S A. 1994; 91:3670–3674.
Article38. Campbell BC, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Yan B, et al. Tenecteplase versus alteplase before endovascular thrombectomy (EXTEND-IA TNK): a multicenter, randomized, controlled study. Int J Stroke. 2018; 13:328–334.39. Macfarlane RG, Pilling J. Fibrinolytic activity of normal urine. Nature. 1947; 159:779.
Article40. Gibson PR, van de Pol E, Doe WF. Cell associated urokinase activity and colonic epithelial cells in health and disease. Gut. 1991; 32:191–195.
Article41. Helenius MA, Saramäki OR, Linja MJ, Tammela TL, Visakorpi T. Amplification of urokinase gene in prostate cancer. Cancer Res. 2001; 61:5340–5344.42. Kusch A, Tkachuk S, Lutter S, Haller H, Dietz R, Lipp M, et al. Monocyte-expressed urokinase regulates human vascular smooth muscle cell migration in a coculture model. Biol Chem. 2002; 383:217–221.
Article43. Medcalf RL, Hamilton JA. Human synovial fibroblasts produce urokinase-type plasminogen activator. Arthritis Rheum. 1986; 29:1397–1401.
Article44. Sidenius N, Blasi F. The urokinase plasminogen activator system in cancer: recent advances and implication for prognosis and therapy. Cancer Metastasis Rev. 2003; 22:205–222.45. Roychoudhury PK, Khaparde SS, Mattiasson B, Kumar A. Synthesis, regulation and production of urokinase using mammalian cell culture: a comprehensive review. Biotechnol Adv. 2006; 24:514–528.
Article46. Collen D, Lijnen HR, Vanlinthout I, Kieckens L, Nelles L, Stassen JM. Thrombolytic and pharmacokinetic properties of human tissue-type plasminogen activator variants, obtained by deletion and/or duplication of structural/functional domains, in a hamster pulmonary embolism model. Thromb Haemost. 1991; 65:174–180.
Article47. Kadir RRA, Bayraktutan U. Urokinase plasminogen activator: a potential thrombolytic agent for ischaemic stroke. Cell Mol Neurobiol. 2020; 40:347–355.
Article48. Bell WR, Simon TL, Stengle JM, Sherry S. The urokinase-streptokinase pulmonary embolism trial (phase II) results. Circulation. 1974; 50:1070–1071.
Article49. Spiecker M, Windeler J, Vermeer F, Michels R, Seabra-Gomes R, vom Dahl J, et al. Thrombolysis with saruplase versus streptokinase in acute myocardial infarction: five-year results of the PRIMI trial. Am Heart J. 1999; 138:518–524.
Article50. Bär FW, Meyer J, Vermeer F, Michels R, Charbonnier B, Haerten K, et al. Comparison of saruplase and alteplase in acute myocardial infarction. SESAM Study Group. The Study in Europe with Saruplase and Alteplase in Myocardial Infarction. Am J Cardiol. 1997; 79:727–732.51. Tillett WS, Garner RL. The fibrinolytic activity of hemolytic streptococci. J Exp Med. 1933; 58:485–502.
Article52. Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, et al. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science. 2004; 305:1283–1286.
Article53. Lack CH. Staphylokinase; an activator of plasma protease. Nature. 1948; 161:559.54. Collen D. Staphylokinase: a potent, uniquely fibrin-selective thrombolytic agent. Nat Med. 1998; 4:279–284.
Article55. Sakharov DV, Lijnen HR, Rijken DC. Interactions between staphylokinase, plasmin(ogen), and fibrin. Staphylokinase discriminates between free plasminogen and plasminogen bound to partially degraded fibrin. J Biol Chem. 1996; 271:27912–27918.56. Armstrong PW, Burton J, Pakola S, Molhoek PG, Betriu A, Tendera M, et al. Collaborative angiographic patency trial of recombinant staphylokinase (CAPTORS II). Am Heart J. 2003; 146:484–488.
Article57. Armstrong PW, Burton JR, Palisaitis D, Thompson CR, Van de Werf F, Rose B, et al. Collaborative angiographic patency trial of recombinant staphylokinase (CAPTORS). Am Heart J. 2000; 139:820–823.
Article58. Cartwright T. The plasminogen activator of vampire bat saliva. Blood. 1974; 43:317–326.
Article59. Krätzschmar J, Haendler B, Langer G, Boidol W, Bringmann P, Alagon A, et al. The plasminogen activator family from the salivary gland of the vampire bat Desmodus rotundus: cloning and expression. Gene. 1991; 105:229–237.60. Bringmann P, Gruber D, Liese A, Toschi L, Krätzchmar J, Schleuning WD, et al. Structural features mediating fibrin selectivity of vampire bat plasminogen activators. J Biol Chem. 1995; 270:25596–25603.
Article61. Kazemali M, Majidzadeh AK, Sardari S, Saadatirad AH, Khalaj V, Zarei N, et al. Design of a novel chimeric tissue plasminogen activator with favorable vampire bat plasminogen activator properties. Enzyme Microb Technol. 2014; 67:82–86.
Article62. Saadatirad A, Sardari S, Kazemali M, Zarei N, Davami F, Barkhordari F, et al. Expression of a novel chimeric-truncated tPA in Pichia pastoris with improved biochemical properties. Mol Biotechnol. 2014; 56:1143–1150.
Article63. Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. The desmoteplase in acute ischemic stroke trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005; 36:66–73.64. Furlan AJ, Eyding D, Albers GW, Al-Rawi Y, Lees KR, Rowley HA, et al. Dose escalation of desmoteplase for acute ischemic stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006; 37:1227–1231.65. Albers GW, von Kummer R, Truelsen T, Jensen JK, Ravn GM, Grønning BA, et al. Safety and efficacy of desmoteplase given 3-9 h after ischaemic stroke in patients with occlusion or high-grade stenosis in major cerebral arteries (DIAS-3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Neurol. 2015; 14:575–584.
Article66. Van de Werf FJ. The ideal fibrinolytic: can drug design improve clinical results? Eur Heart J. 1999; 20:1452–1458.
Article67. Tennant SN, Dixon J, Venable TC, Page HL Jr, Roach A, Kaiser AB, et al. Intracoronary thrombolysis in patients with acute myocardial infarction: comparison of the efficacy of urokinase with streptokinase. Circulation. 1984; 69:756–760.
Article68. Marini C, Di Ricco G, Rossi G, Rindi M, Palla R, Giuntini C. Fibrinolytic effects of urokinase and heparin in acute pulmonary embolism: a randomized clinical trial. Respiration. 1988; 54:162–173.
Article69. Van Hulle F, Bonkain F, De Clerck D, Aerden D, Vanwijn I, Tielemans C, et al. Efficacy of urokinase lock to treat thrombotic dysfunction of tunneled hemodialysis catheters: a retrospective cohort study. J Vasc Access. 2019; 20:60–69.
Article70. Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982; 257:2912–2919.
Article71. Urano T, Castellino FJ, Suzuki Y. Regulation of plasminogen activation on cell surfaces and fibrin. J Thromb Haemost. 2018; 16:1487–1497.
Article72. Collen D, Lijnen HR. The tissue-type plasminogen activator story. Arterioscler Thromb Vasc Biol. 2009; 29:1151–1155.
Article73. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018; 378:1573–1582.74. Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010; 41:2254–2258.75. Bivard A, Huang X, Levi CR, Spratt N, Campbell BCV, Cheripelli BK, et al. Tenecteplase in ischemic stroke offers improved recanalization: analysis of 2 trials. Neurology. 2017; 89:62–67.76. Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017; 48:e343–e361.
Article77. National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333:1581–1587.78. Mair G, von Kummer R, Adami A, White PM, Adams ME, Yan B, et al. Arterial obstruction on computed tomographic or magnetic resonance angiography and response to intravenous thrombolytics in ischemic stroke. Stroke. 2017; 48:353–360.
Article79. Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011; 42:1775–1777.80. Menon BK, Al-Ajlan FS, Najm M, Puig J, Castellanos M, Dowlatshahi D, et al. Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA. 2018; 320:1017–1026.
Article81. Zhang Y, Gladysheva IP, Houng AK, Reed GL. Streptococcus uberis plasminogen activator (SUPA) activates human plasminogen through novel species-specific and fibrin-targeted mechanisms. J Biol Chem. 2012; 287:19171–19176.
Article82. Hu Y, Yu D, Wang Z, Hou J, Tyagi R, Liang Y, et al. Purification and characterization of a novel, highly potent fibrinolytic enzyme from Bacillus subtilis DC27 screened from Douchi, a traditional Chinese fermented soybean food. Sci Rep. 2019; 9:9235.
Article83. Khursade PS, Galande SH, Shiva Krishna P, Prakasham RS. Stenotrophomonas maltophilia Gd2: a potential and novel isolate for fibrinolytic enzyme production. Saudi J Biol Sci. 2019; 26:1567–1575.
Article84. Chen H, McGowan EM, Ren N, Lal S, Nassif N, Shad-Kaneez F, et al. Nattokinase: a promising alternative in prevention and treatment of cardiovascular diseases. Biomark Insights. 2018; 13:1177271918785130.
Article85. Silva PEDCE, Barros RC, Albuquerque WWC, Brandão RMP, Bezerra RP, Porto ALF. In vitro thrombolytic activity of a purified fibrinolytic enzyme from Chlorella vulgaris. J Chromatogr B Analyt Technol Biomed Life Sci. 2018; 1092:524–529.
Article86. He J, Chen S, Gu J. Identification and characterization of Harobin, a novel fibrino(geno)lytic serine protease from a sea snake (Lapemis hardwickii). FEBS Lett. 2007; 581:2965–2973.
Article87. Li Z, Chen X, Guo S, Zhang H, Dong H, Wu G, et al. Engineering of Harobin for enhanced fibrinolytic activity obtained by random and site-directed mutagenesis. Protein Expr Purif. 2017; 129:162–172.
Article88. Kim HJ, Shim KH, Yeon SJ, Shin HS. A novel thrombolytic and anticoagulant serine protease from Polychaeta, Diopatra sugokai. J Microbiol Biotechnol. 2018; 28:275–283.
Article89. Vanacek P, Sebestova E, Babkova P, Bidmanova S, Daniel L, Dvorak P, et al. Exploration of enzyme diversity by integrating bioinformatics with expression analysis and biochemical characterization. ACS Catal. 2018; 8:2402–2412.
Article90. Suzuki E, Nishimura N, Yoshikawa T, Kunikiyo Y, Hasegawa K, Hasumi K. Efficacy of SMTP-7, a small-molecule anti-inflammatory thrombolytic, in embolic stroke in monkeys. Pharmacol Res Perspect. 2018; 6:e00448.91. van Zonneveld AJ, Veerman H, Pannekoek H. Autonomous functions of structural domains on human tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1986; 83:4670–4674.
Article92. Medcalf RL. Fibrinolysis: from blood to the brain. J Thromb Haemost. 2017; 15:2089–2098.
Article93. Niego B, Freeman R, Puschmann TB, Turnley AM, Medcalf RL. t-PA-specific modulation of a human blood-brain barrier model involves plasmin-mediated activation of the Rho kinase pathway in astrocytes. Blood. 2012; 119:4752–4761.
Article94. Thiebaut AM, Gauberti M, Ali C, Martinez De Lizarrondo S, Vivien D, Yepes M, et al. The role of plasminogen activators in stroke treatment: fibrinolysis and beyond. Lancet Neurol. 2018; 17:1121–1132.
Article95. Horn IR, van den Berg BM, Moestrup SK, Pannekoek H, van Zonneveld AJ. Plasminogen activator inhibitor 1 contains a cryptic high affinity receptor binding site that is exposed upon complex formation with tissue-type plasminogen activator. Thromb Haemost. 1998; 80:822–828.96. Cole ES, Nichols EH, Poisson L, Harnois ML, Livingston DJ. In vivo clearance of tissue plasminogen activator: the complex role of sites of glycosylation and level of sialylation. Fibrinolysis. 1993; 7:15–22.
Article97. Mohammadi E, Seyedhosseini-Ghaheh H, Mahnam K, Jahanian-Najafabadi A, Sadeghi HMM. Reteplase: structure, function, and production. Adv Biomed Res. 2019; 8:19.
Article98. Maheshwari N, Kantipudi S, Maheshwari A, Arora K, Kwatra N, et al. Amino-terminal fusion of epidermal growth factor 4,5,6 domains of human thrombomodulin on streptokinase confers anti-reocclusion characteristics along with plasmin-mediated clot specificity. PLoS One. 2016; 11:e0150315.
Article99. Taheri MN, Behzad-Behbahani A, Rafiei Dehbidi G, Salehi S, Sharifzadeh S. Engineering, expression and purification of a chimeric fibrin-specific streptokinase. Protein Expr Purif. 2016; 128:14–21.
Article100. He J, Di J, Xu R, Zhao B. Novel recombinant thrombolytic and antithrombotic staphylokinase variants with an RGD motif at their N-termini. Biotechnol Appl Biochem. 2008; 50:17–23.
Article101. Szemraj J, Walkowiak B, Kawecka I, Janiszewska G, Buczko W, Bartkowiak J, et al. A new recombinant thrombolytic and antithrombotic agent with higher fibrin affinity: a staphylokinase variant. I. In vitro study. J Thromb Haemost. 2005; 3:2156–2165.102. Chiou JF, Woon MD, Cheng SN, Hsu CH, Cherng SC, Hsieh FK, et al. Staphylokinase-annexin XI chimera exhibited efficient in vitro thrombolytic activities. Biosci Biotechnol Biochem. 2007; 71:1122–1129.103. Faraji H, Ramezani M, Mashkani B, Sadeghnia HR, Benhangi HM, Hosseini Teshnizi S, et al. Comparison of expression optimization of new derivative of staphylokinase (SAK-2RGDTTI) with the rSAK. Biotechnol Prog. 2019; 35:e2819.
Article104. Wu SC, Castellino FJ, Wong SL. A fast-acting, modular-structured staphylokinase fusion with kringle-1 from human plasminogen as the fibrin-targeting domain offers improved clot lysis efficacy. J Biol Chem. 2003; 278:18199–18206.
Article105. Raigani M, Rouini MR, Golabchifar AA, Mirabzadeh E, Vaziri B, Barkhordari F, et al. Scale up and pharmacokinetic study of a novel mutated chimeric tissue plasminogen activator (mt-PA) in rats. Sci Rep. 2017; 7:43028.
Article106. Davami F, Sardari S, Majidzadeh AK, Hemayatkar M, Barkhrdari F, Omidi M, et al. Expression of a novel chimeric truncated t-PA in CHO cells based on in silico experiments. J Biomed Biotechnol. 2010; 2010:108159.
Article107. Bonnard T, Tennant Z, Niego B, Kanojia R, Alt K, Jagdale S, et al. Novel thrombolytic drug based on thrombin cleavable microplasminogen coupled to a single-chain antibody specific for activated GPIIb/IIIa. J Am Heart Assoc. 2017; 6:e004535.
Article108. Wu XC, Ye R, Duan Y, Wong SL. Engineering of plasmin-resistant forms of streptokinase and their production in Bacillus subtilis: streptokinase with longer functional half-life. Appl Environ Microbiol. 1998; 64:824–829.109. Babbal A, Mohanty S, Khasa YP. Engineering of deglycosylated and plasmin resistant variants of recombinant streptokinase in Pichia pastoris. Appl Microbiol Biotechnol. 2018; 102:10561–10577.
Article110. Sawhney P, Katare K, Sahni G. PEGylation of truncated streptokinase leads to formulation of a useful drug with ameliorated attributes. PLoS One. 2016; 11:e0155831.
Article111. Xu Y, Shi Y, Zhou J, Yang W, Bai L, Wang S, et al. Structure-based antigenic epitope and PEGylation improve the efficacy of staphylokinase. Microb Cell Fact. 2017; 16:197.
Article112. Collen D. Engineered staphylokinase variants with reduced immunogenicity. Fibrinolysis Proteolysis. 1998; 12 Suppl 2:59–65.
Article113. Thomas GR, Thibodeaux H, Errett CJ, Badillo JM, Keyt BA, Refino CJ, et al. A long-half-life and fibrin-specific form of tissue plasminogen activator in rabbit models of embolic stroke and peripheral bleeding. Stroke. 1994; 25:2072–2078.
Article114. Armstead WM, Riley J, Yarovoi S, Cines DB, Smith DH, Higazi AA. tPA-S481A prevents neurotoxicity of endogenous tPA in traumatic brain injury. J Neurotrauma. 2012; 29:1794–1802.
Article115. Goulay R, Naveau M, Gaberel T, Vivien D, Parcq J. Optimized tPA: a non-neurotoxic fibrinolytic agent for the drainage of intracerebral hemorrhages. J Cereb Blood Flow Metab. 2018; 38:1180–1189.
Article116. Gurewich V, Pannell R, Simmons-Byrd A, Sarmientos P, Liu JN, Badylak SF. Thrombolysis vs. bleeding from hemostatic sites by a prourokinase mutant compared with tissue plasminogen activator. J Thromb Haemost. 2006; 4:1559–1565.
Article117. Koudelka S, Mikulik R, Masek J, Raska M, Turanek Knotigova P, Miller AD, et al. Liposomal nanocarriers for plasminogen activators. J Control Release. 2016; 227:45–57.
Article118. Bruch GE, Fernandes LF, Bassi BLT, Alves MTR, Pereira IO, Frezard F, et al. Liposomes for drug delivery in stroke. Brain Res Bull. 2019; 152:246–256.
Article119. Kim JY, Kim JK, Park JS, Byun Y, Kim CK. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials. 2009; 30:5751–5756.
Article120. Al-Ahmady ZS, Jasim D, Ahmad SS, Wong R, Haley M, Coutts G, et al. Selective liposomal transport through blood brain barrier disruption in ischemic stroke reveals two distinct therapeutic opportunities. ACS Nano. 2019; 13:12470–12486.
Article121. Partoazar A, Nasoohi S, Rezayat SM, Gilani K, Mehr SE, Amani A, et al. Nanoliposome containing cyclosporine A reduced neuroinflammation responses and improved neurological activities in cerebral ischemia/reperfusion in rat. Fundam Clin Pharmacol. 2017; 31:185–193.
Article122. Tiebosch IA, Crielaard BJ, Bouts MJ, Zwartbol R, Salas-Perdomo A, Lammers T, et al. Combined treatment with recombinant tissue plasminogen activator and dexamethasone phosphate-containing liposomes improves neurological outcome and restricts lesion progression after embolic stroke in rats. J Neurochem. 2012; 123:65–74.
Article123. Absar S, Gupta N, Nahar K, Ahsan F. Engineering of plasminogen activators for targeting to thrombus and heightening thrombolytic efficacy. J Thromb Haemost. 2015; 13:1545–1556.
Article124. Zhang N, Li C, Zhou D, Ding C, Jin Y, Tian Q, et al. Cyclic RGD functionalized liposomes encapsulating urokinase for thrombolysis. Acta Biomater. 2018; 70:227–236.
Article125. Laing ST, Moody M, Smulevitz B, Kim H, Kee P, Huang S, et al. Ultrasound-enhanced thrombolytic effect of tissue plasminogen activator-loaded echogenic liposomes in an in vivo rabbit aorta thrombus model-brief report. Arterioscler Thromb Vasc Biol. 2011; 31:1357–1359.
Article126. Korin N, Gounis MJ, Wakhloo AK, Ingber DE. Targeted drug delivery to flow-obstructed blood vessels using mechanically activated nanotherapeutics. JAMA Neurol. 2015; 72:119–122.
Article127. Saxena V, Gacchina Johnson C, Negussie AH, Sharma KV, Dreher MR, Wood BJ. Temperature-sensitive liposome-mediated delivery of thrombolytic agents. Int J Hyperthermia. 2015; 31:67–73.
Article128. Xu J, Zhang Y, Xu J, Liu G, Di C, Zhao X, et al. Engineered nanoplatelets for targeted delivery of plasminogen activators to reverse thrombus in multiple mouse thrombosis models. Adv Mater. 2020; 32:e1905145.
Article129. Chen K, Wang Y, Liang H, Xia S, Liang W, Kong J, et al. Intrinsic biotaxi solution based on blood cell membrane cloaking enables fullerenol thrombolysis in vivo. ACS Appl Mater Interfaces. 2020; 12:14958–14970.
Article130. Wang X, Lin X, Loy JA, Tang J, Zhang XC. Crystal structure of the catalytic domain of human plasmin complexed with streptokinase. Science. 1998; 281:1662–1665.
Article131. Rehan M, Sagar A, Sharma V, Mishra S, Sahni G. Penta-l-lysine potentiates fibrin-independent activity of human tissue plasminogen activator. J Phys Chem B. 2015; 119:13271–13277.
Article132. Ghaheh HS, Ganjalikhany MR, Yaghmaei P, Pourfarzam M, Sadeghi HMM. Improving the solubility, activity, and stability of reteplase using in silico design of new variants. Research in pharmaceutical sciences. 2019; 14:359–368.133. Krishnamurthy A, Belur PD, Subramanya SB. Methods available to assess therapeutic potential of fibrinolytic enzymes of microbial origin: a review. J Anal Sci Technol. 2018; 9:10.
Article134. Kotb E. Activity assessment of microbial fibrinolytic enzymes. Appl Microbiol Biotechnol. 2013; 97:6647–6665.
Article135. Millar WT, Smith JF. A highly sensitive solid phase assay for plasminogen activators using 125I-fibrinogen. Thromb Res. 1978; 13:389–395.
Article136. Millar WT, Smith JF. The comparison of solid phase and fibrin plate methods for the measurement of plasminogen activators. Thromb Res. 1983; 30:431–439.
Article137. Hume M. Radioactive (I125) human plasma clots for assay of thrombolytic activity. J Lab Clin Med. 1964; 63:699–702.138. Moroz LA, Gilmore NJ. A rapid and sensitive 125I-fibrin solid-phase fibrinolytic assay for plasmin. Blood. 1975; 46:543–553.
Article139. Loskutoff DJ. Effect of thrombin on the fibrinolytic activity of cultured bovine endothelial cells. J Clin Invest. 1979; 64:329–332.
Article140. Roche PC, Campeau JD, Shaw ST. A rapid and highly sensitive solid-phase radioassay for plasminogen activators. Thromb Res. 1983; 31:269–277.
Article141. Gidron E, Margalit R, Shalitin Y. A rapid screening test for reduced fibrinolytic activity of plasma: streptokinase activated lysis time. J Clin Pathol. 1978; 31:54–57.
Article142. Patchett SE, Enright H, Afdhal N, O’Connell W, O’Donoghue DP. Clot lysis by gastric juice: an in vitro study. Gut. 1989; 30:1704–1707.
Article143. Goldenberg NA, Hathaway WE, Jacobson L, Manco-Johnson M. A new global assay of coagulation and fibrinolysis. J Thromb Res. 2005; 116:345–356.
Article144. Astrup T, Mullertz S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952; 40:346–351.
Article145. Jespersen J, Astrup T. A study of the fibrin plate assay of fibrinolytic agents. Optimal conditions, reproducibility and precision. Haemostasis. 1983; 13:301–315.146. Marsh NA, Gaffney PJ. The rapid fibrin plate: a method for plasminogen activator assay. J Thromb Haemost. 1977; 38:545–551.147. Lassen M. Heat denaturation of plasminogen in the fibrin plate method. Acta Physiol Scand. 1953; 27:371–376.148. Barta G. Dyed fibrin plate assay of fibrinolysis. Can J Physiol Pharmacol. 1966; 44:233–240.
Article149. Fossum S, Hoem NO. Urokinase and non-urokinase fibrinolytic activity in protease-inhibitor-deprived plasma, assayed by a fibrin micro-plate method. Immunopharmacology. 1996; 32:119–121.
Article150. Sherry S, Alkjaersig N, Fletcher AP. Activity of plasmin and streptokinase-activator on substituted arginine and lysine esters. Thromb Diath Haemorrh. 1966; 16:18–31.
Article151. Niinobe M, Hitomi Y, Fujii S. A sensitive colorimetric assay for various proteases using naphthyl ester derivatives as substrates. J Biochem. 1980; 87:779–783.
Article152. Barlow GH, Marder VJ. Plasma urokinase levels measured by chromogenic assay after infusions of tissue culture or urinary source material. Thromb Res. 1980; 18:431–437.
Article153. Kulseth MA, Helgeland L. A highly sensitive chromogenic microplate assay for quantification of rat and human plasminogen. Anal Biochem. 1993; 210:314–317.
Article154. Nieuwenhuizen W, Wijngaards G, Groeneveld E. Fluorogenic substrates for sensitive and differential estimation of urokinase and tissue plasminogen activator. Haemostasis. 1978; 7:146–149.
Article155. Zimmerman M. Direct fluorescent assay of urokinase and plasminogen activators of normal and malignant cells: kinetics and inhibitor profiles. Proc Natl Acad Sci U S A. 1978; 75:750–753.
Article156. Mander P, Cho SS, Simkhada JR, Choi YH, Yoo JC. A low molecular weight chymotrypsin-like novel fibrinolytic enzyme from Streptomyces sp. CS624. Process Biochem. 2011; 46:1449–1455.
Article157. Salazar AM, Rodriguez-Acosta A, Girón ME, Aguilar I, Guerrero B. A comparative analysis of the clotting and fibrinolytic activities of the snake venom (Bothrops atrox) from different geographical areas in Venezuela. Thromb Res. 2007; 120:95–104.
Article158. Stewart RJ. Identification of the mechanism responsible for the increased fibrin specificity of TNK-tissue plasminogen activator relative to tissue plasminogen activator. J Biol Chem. 2000; 275:10112–10120.
Article159. Radcliffe R, Heinze T. Stimulation of tissue plasminogen activator by denatured proteins and fibrin clots: a possible additional role for plasminogen activator? Arch Biochem Biophys. 1981; 211:750–761.
Article160. Longstaff C, Whitton CM. A proposed reference method for plasminogen activators that enables calculation of enzyme activities in SI units. Thromb Haemost. 2004; 2:1416–1421.
Article161. Stewart RJ, Fredenburgh JC, Weitz JI. Characterization of the interactions of plasminogen and tissue and vampire bat plasminogen activators with fibrinogen, fibrin, and the complex of d-Dimer noncovalently linked to fragment E. J Biol Chem. 1998; 273:18292–18299.
Article162. Hawkey CM, Stafford JL. A standard clot method for the assay of plasminogen activators, anti-activators, and plasmin. J Clin Pathol. 1964; 17:175–181.
Article163. Somerville DA. A technique for demonstrating fibrinolysis by cutaneous bacteria. J Clin Pathol. 1972; 25:740–741.
Article164. Chakrabarti R, Fearnley GR. The ‘fibrinolytic potential’ as a simple measure of spontaneous fibrinolysis. J Clin Pathol. 1962; 15:228–230.
Article165. Blix S. The effectiveness of activators in clot lysis, with special reference to fibrinolytic therapy: a new method for determination of preformed clot lysis. Acta Med Scand Suppl. 1962; 386:1–24.
Article166. Howell M. A method for assessing clot lysis. J Clin Pathol. 1964; 17:310–312.
Article167. Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM, Daginawala HF. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb J. 2006; 4:14.
Article168. Narasimhan MK, Chandrasekaran M, Rajesh M. Fibrinolytic enzyme production by newly isolated Bacillus cereus SRM-001 with enhanced in-vitro blood clot lysis potential. J Gen Appl Microbiol. 2015; 61:157–164.169. Smith AA, Jacobson LJ, Miller BI, Hathaway WE, Manco-Johnson M. A new euglobulin clot lysis assay for global fibrinolysis. J Thromb Res. 2003; 112:329–337.
Article170. He S, Bremme K, Blombäck M. A laboratory method for determination of overall haemostatic potential in plasma. I. Method design and preliminary results. Thromb Res. 1999; 96:145–156.
Article171. Ilich A. Development and application of global assays of hyper- and hypofibrinolysis. Res Pract Thromb Haemost. 2020; 4:46–53.
Article172. Zamanlu M, Eskandani M, Mohammadian R, Entekhabi N, Rafi M, Farhoudi M. Spectrophotometric analysis of thrombolytic activity: SATA assay. Bioimpacts. 2018; 8:31–38.
Article173. Bonnard T, Law LS, Tennant Z, Hagemeyer CE. Development and validation of a high throughput whole blood thrombolysis plate assay. Sci Rep. 2017; 7:2346.
Article174. Donahue SM, Otto CM. Thromboelastography: a tool for measuring hypercoagulability, hypocoagulability, and fibrinolysis. J Vet Emerg Crit Care. 2005; 15:9–16.
Article175. Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev. 2012; 26:1–13.
Article176. Fan NK, Keegan PM, Platt MO, Averett RD. Experimental and imaging techniques for examining fibrin clot structures in normal and diseased states. J Vis Exp. 2015; 98:e52019.
Article177. Kim DE, Kim JY, Lee SK, Ryu JH, Kwon IC, Ahn CH, et al. Combined near-infrared fluorescent imaging and micro-computed tomography for directly visualizing cerebral thromboemboli. J Vis Exp. 2016; 54294.
Article178. Davami F, Sardari S, Majidzadeh AK, Hemayatkar M, Barkhordari F, Enayati S, et al. A novel variant of t-PA resistant to plasminogen activator inhibitor-1: expression in CHO cells based on in silico experiments. BMB Rep. 2011; 44:34–39.
Article179. Hekman CM, Loskutoff DJ. Kinetic analysis of the interactions between plasminogen activator inhibitor 1 and both urokinase and tissue plasminogen activator. Arch Biochem Biophys. 1988; 262:199–210.
Article180. Chmielewska J, Rånby M, Wiman B. Evidence for a rapid inhibitor to tissue plasminogen activator in plasma. Thromb Res. 1983; 31:427–436.
Article181. Franke AE, Danley DE, Kaczmarek FS, Hawrylik SJ, Gerard RD, Lee SE, et al. Expression of human plasminogen activator inhibitor type-1 (PAI-1) in Escherichia coli as a soluble protein comprised of active and latent forms. Isolation and crystallization of latent PAI-1. Biochim Biophys Acta. 1990; 1037:16–23.
Article182. Wind T, Jensen MA, Andreasen PA. Epitope mapping for four monoclonal antibodies against human plasminogen activator inhibitor type-1: implications for antibody-mediated PAI-1-neutralization and vitronectin-binding. Eur J Biochem. 2001; 268:1095–1106.183. Yakovlev S, Makogonenko E, Kurochkina N, Nieuwenhuizen W, Ingham K, Medved L. Conversion of fibrinogen to fibrin: mechanism of exposure of tPA- and plasminogen-binding sites. Biochemistry. 2000; 39:15730–15741.
Article184. Tsurupa G, Medved L. Identification and characterization of novel tPA- and plasminogen-binding sites within fibrin(ogen) alpha C-domains. Biochemistry. 2001; 40:801–808.185. Kim PY, Tieu LD, Stafford AR, Fredenburgh JC, Weitz JI. A high affinity interaction of plasminogen with fibrin is not essential for efficient activation by tissue plasminogen activator. J Biol Chem. 2012; 287:4652–4661.186. Tsurupa G, Medved L. Fibrinogen alpha C domains contain cryptic plasminogen and tPA binding sites. Ann N Y Acad Sci. 2001; 936:328–330.187. de Munk GA, Caspers MP, Chang GT, Pouwels PH, Enger-Valk BE, Verheijen JH. Binding of tissue-type plasminogen activator to lysine, lysine analogues, and fibrin fragments. Biochemistry. 1989; 28:7318–7325.188. Jiao J, Yu M, Ru B. Characterization of a recombinant chimeric plasminogen activator with enhanced fibrin binding. Enzymology. 2001; 1546:399–405.
Article189. Higgins DL, Vehar GA. Interaction of one-chain and twochain tissue plasminogen activator with intact and plasmin-degraded fibrin. Biochemistry. 1987; 26:7786–7791.
Article190. Verheijen JH, Caspers MP, Chang GT, de Munk GA, Pouwels PH, Enger-Valk BE. Involvement of finger domain and kringle 2 domain of tissue-type plasminogen activator in fibrin binding and stimulation of activity by fibrin. EMBO J. 1986; 5:3525–3530.
Article191. Xu Z, Balsara RD, Gorlatova NV, Lawrence DA, Castellino FJ, Ploplis VA. Conservation of critical functional domains in murine plasminogen activator inhibitor-1. J Biol Chem. 2004; 279:17914–17920.
Article192. Andersen OM, Petersen HH, Jacobsen C, Moestrup SK, Etzerodt M, Andreasen PA, et al. Analysis of a two-domain binding site for the urokinase-type plasminogen activator-plasminogen activator inhibitor-1 complex in low-density-lipoprotein-receptor-related protein. Biochem J. 2001; 357:289–296.
Article193. Sánchez MC, Chiabrando GA, Vides MA. Pregnancy zone protein-tissue-type plasminogen activator complexes bind to low-density lipoprotein receptor-related protein (LRP). Arch Biochem Biophys. 2001; 389:218–222.
Article194. Dolmer K, Campos A, Gettins PG. Quantitative dissection of the binding contributions of ligand lysines of the receptor-associated protein (RAP) to the low density lipoprotein receptor-related protein (LRP1). J Biol Chem. 2013; 288:24081–24090.
Article195. Jensen JK, Dolmer K, Schar C, Gettins PGW. Receptor-associated protein (RAP) has two high-affinity binding sites for the low-density lipoprotein receptor-related protein (LRP): consequences for the chaperone functions of RAP. Biochem J. 2009; 421:273–282.
Article196. Asahi M, Rammohan R, Sumii T, Wang X, Pauw RJ, Weissig V, et al. Antiactin-targeted immunoliposomes ameliorate tissue plasminogen activator-induced hemorrhage after focal embolic stroke. J Cereb Blood Flow Metab. 2003; 23:895–899.
Article197. Esmaeeli-Nadimi A, Kennedy D, Allahtavakoli M. Opening the window: ischemic postconditioning reduces the hyperemic response of delayed tissue plasminogen activator and extends its therapeutic time window in an embolic stroke model. Eur J Pharmacol. 2015; 764:55–62.
Article198. Hao CH, Ding WX, Sun Q, Li XX, Wang WT, Zhao ZY, et al. Thrombolysis with rhPro-UK 3 to 6 hours after embolic stroke in rat. Neurol Res. 2019; 41:1034–1042.
Article199. Ishrat T, Fouda AY, Pillai B, Eldahshan W, Ahmed H, Waller JL, et al. Dose-response, therapeutic time-window and tPA-combinatorial efficacy of compound 21: a randomized, blinded preclinical trial in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab. 2019; 39:1635–1647.
Article200. Jin R, Xiao AY, Liu S, Wang M, Li G. Taurine reduces tPA (tissue-type plasminogen activator)-induced hemorrhage and microvascular thrombosis after embolic stroke in rat. Stroke. 2018; 49:1708–1718.
Article201. Jin R, Zhu X, Li G. Embolic middle cerebral artery occlusion (MCAO) for ischemic stroke with homologous blood clots in rats. J Vis Exp. 2014; 91:51956.
Article202. Kudo M, Aoyama A, Ichimori S, Fukunaga N. An animal model of cerebral infarction. Homologous blood clot emboli in rats. Stroke. 1982; 13:505–508.
Article203. Lee KY, Bae ON, Weinstock S, Kassab M, Majid A. Neuroprotective effect of asiatic acid in rat model of focal embolic stroke. Biol Pharm Bull. 2014; 37:1397–1401.
Article204. Lehmann J, Hartig W, Seidel A, Fuldner C, Hobohm C, Grosche J, et al. Inflammatory cell recruitment after experimental thromboembolic stroke in rats. Neuroscience. 2014; 279:139–154.
Article205. Ren M, Lin ZJ, Qian H, Choudhury GR, Liu R, Liu H, et al. Embolic middle cerebral artery occlusion model using thrombin and fibrinogen composed clots in rat. J Neurosci Methods. 2012; 211:296–304.
Article206. Shimamura N, Matsuda N, Kakuta K, Narita A, Ohkuma H. A model of rat embolic cerebral infarction with a quantifiable, autologous arterial blood clot. Transl Stroke Res. 2013; 4:564–570.
Article207. Si Z, Liu J, Hu K, Lin Y, Liu J, Wang A. Effects of thrombolysis within 6 hours on acute cerebral infarction in an improved rat embolic middle cerebral artery occlusion model for ischaemic stroke. J Cell Mol Med. 2019; 23:2468–2474.208. Takano K, Carano RA, Tatlisumak T, Meiler M, Sotak CH, Kleinert HD, et al. Efficacy of intra-arterial and intravenous prourokinase in an embolic stroke model evaluated by diffusion-perfusion magnetic resonance imaging. Neurology. 1998; 50:870–875.
Article209. Zhang L, Chopp M, Jia L, Cui Y, Lu M, Zhang ZG. Atorvastatin extends the therapeutic window for tPA to 6 h after the onset of embolic stroke in rats. J Cereb Blood Flow Metab. 2009; 29:1816–1824.
Article210. Zhang L, Chopp M, Lu M, Zhang T, Li C, Winter S, et al. Demonstration of therapeutic window of cerebrolysin in embolic stroke: a prospective, randomized, blinded, and placebo-controlled study. Int J Stroke. 2017; 12:628–635.
Article211. Zhang L, Chopp M, Meier DH, Winter S, Wang L, Szalad A, et al. Sonic hedgehog signaling pathway mediates cerebrolysin-improved neurological function after stroke. Stroke. 2013; 44:1965–1972.
Article212. Zhang L, Zhang RL, Jiang Q, Ding G, Chopp M, Zhang ZG. Focal embolic cerebral ischemia in the rat. Nat Protoc. 2015; 10:539–547.
Article213. Chen S, Chen Z, Cui J, McCrary ML, Song H, Mobashery S, et al. Early abrogation of gelatinase activity extends the time window for tPA thrombolysis after embolic focal cerebral ischemia in mice. eNeuro. 2018; 5:ENEURO.0391–17.2018.
Article214. Huang L, Wang J, Huang S, Siaw-Debrah F, Nyanzu M, Zhuge Q. Polyacrylic acid-coated nanoparticles loaded with recombinant tissue plasminogen activator for the treatment of mice with ischemic stroke. Biochem Biophys Res Commun. 2019; 516:565–570.
Article215. Kanazawa M, Kawamura K, Takahashi T, Miura M, Tanaka Y, Koyama M, et al. Multiple therapeutic effects of progranulin on experimental acute ischaemic stroke. Brain. 2015; 138:1932–1948.
Article216. Kim DE, Kim JY, Nahrendorf M, Lee SK, Ryu JH, Kim K, et al. Direct thrombus imaging as a means to control the variability of mouse embolic infarct models: the role of optical molecular imaging. Stroke. 2011; 42:3566–3573.217. Kim JY, Ryu JH, Schellingerhout D, Sun IC, Lee SK, Jeon S, et al. Direct imaging of cerebral thromboemboli using computed tomography and fibrin-targeted gold nanoparticles. Theranostics. 2015; 5:1098–1114.
Article218. Sladojevic N, Oh GT, Kim HH, Beaulieu LM, Falet H, Kaminski K, et al. Decreased thromboembolic stroke but not atherosclerosis or vascular remodelling in mice with ROCK2-deficient platelets. Cardiovasc Res. 2017; 113:1307–1317.
Article219. Zhu YQ, Zhao MY, Gu XC, Teng GJ. Evaluation of neurovascular function in mouse cortex using multispectral optical imaging after ischemic stroke. Zhonghua Yi Xue Za Zhi. 2019; 99:2943–2946.220. Culp WC, Woods SD, Skinner RD, Brown AT, Lowery JD, Johnson JL, et al. Dodecafluoropentane emulsion decreases infarct volume in a rabbit ischemic stroke model. J Vasc Interv Radiol. 2012; 23:116–121.
Article221. Jahan R, Villablanca JP, Harris RJ, Duarte-Vogel S, Williams CK, Vinters HV, et al. Selective middle cerebral artery occlusion in the rabbit: technique and characterization with pathologic findings and multimodal MRI. J Neurosci Methods. 2019; 313:6–12.
Article222. Lapchak PA, Araujo DM, Pakola S, Song D, Wei J, Zivin JA. Microplasmin: a novel thrombolytic that improves behavioral outcome after embolic strokes in rabbits. Stroke. 2002; 33:2279–2284.
Article223. Lapchak PA, Araujo DM, Song D, Wei J, Purdy R, Zivin JA. Effects of the spin trap agent disodium-[tert-butylimino) methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) on intracerebral hemorrhage in a rabbit large clot embolic stroke model: combination studies with tissue plasminogen activator. Stroke. 2002; 33:1665–1670.224. Lapchak PA, Araujo DM, Song D, Wei J, Zivin JA. Neuroprotective effects of the spin trap agent disodium-[(tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) in a rabbit small clot embolic stroke model: combination studies with the thrombolytic tissue plasminogen activator. Stroke. 2002; 33:1411–1415.225. Liu S, Xu X, Cheng Q, Zu Q, Lu S, Yu J, et al. Simple quantitative measurement based on DWI to objectively judge DWI-FLAIR mismatch in a canine stroke model. Diagn Interv Radiol. 2015; 21:348–354.
Article226. Xu XQ, Cheng QG, Zu QQ, Lu SS, Yu J, Sheng Y, et al. Comparative study of the relative signal intensity on DWI, FLAIR, and T2 images in identifying the onset time of stroke in an embolic canine model. Neurol Sci. 2014; 35:1059–1065.
Article227. Lee IJ, Yang YC, Hsu JW, Chang WT, Chuang YJ, Liau I. Zebrafish model of photochemical thrombosis for translational research and thrombolytic screening in vivo. J Biophotonics. 2017; 10:494–502.
Article228. Kito G, Nishimura A, Susumu T, Nagata R, Kuge Y, Yokota C, et al. Experimental thromboembolic stroke in cynomolgus monkey. J Neurosci Methods. 2001; 105:45–53.
Article229. Kuge Y, Yokota C, Tagaya M, Hasegawa Y, Nishimura A, Kito G, et al. Serial changes in cerebral blood flow and flow-metabolism uncoupling in primates with acute thromboembolic stroke. J Cereb Blood Flow Metab. 2001; 21:202–210.
Article230. Ansar S, Chatzikonstantinou E, Wistuba-Schier A, Mirau-Weber S, Fatar M, Hennerici MG, et al. Characterization of a new model of thromboembolic stroke in C57 black/6J mice. Transl Stroke Res. 2014; 5:526–533.
Article231. Zhang Z, Zhang RL, Jiang Q, Raman SB, Cantwell L, Chopp M. A new rat model of thrombotic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997; 17:123–135.
Article232. Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007; 38:2771–2778.
Article233. Beech JS, Williams SC, Campbell CA, Bath PM, Parsons AA, Hunter AJ, et al. Further characterisation of a thromboembolic model of stroke in the rat. Brain Res. 2001; 895:18–24.
Article234. Atochin DN, Murciano JC, Gursoy-Ozdemir Y, Krasik T, Noda F, Ayata C, et al. Mouse model of microembolic stroke and reperfusion. Stroke. 2004; 35:2177–2182.
Article235. Agren A, Edgren G, Kardell M, Ostlund A, Wikman AT. In vitro combinations of red blood cell, plasma and platelet components evaluated by thromboelastography. Blood Transfus. 2014; 12:491–496.236. Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997; 766:83–92.
Article237. Kim DE, Kim JY, Schellingerhout D, Ryu JH, Lee SK, Jeon S, et al. Quantitative imaging of cerebral thromboemboli in vivo: the effects of tissue-type plasminogen activator. Stroke. 2017; 48:1376–1385.238. Chen Y, Zhu W, Zhang W, Libal N, Murphy SJ, Offner H, et al. A novel mouse model of thromboembolic stroke. J Neurosci Methods. 2015; 256:203–211.
Article239. Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005; 36:1432–1438.240. Mehta BP, Nogueira RG. Should clot composition affect choice of endovascular therapy? Neurology. 2012; 79:S63–67.
Article241. Gauberti M, Martinez de Lizarrondo S, Orset C, Vivien D. Lack of secondary microthrombosis after thrombin-induced stroke in mice and non-human primates. J Thromb Haemost. 2014; 12:409–414.
Article242. Orset C, Haelewyn B, Allan SM, Ansar S, Campos F, Cho TH, et al. Efficacy of alteplase in a mouse model of acute ischemic stroke: a retrospective pooled analysis. Stroke. 2016; 47:1312–1318.243. Tseng MT, Dozier A, Haribabu B, Graham UM. Transendothelial migration of ferric ion in FeCl3 injured murine common carotid artery. Thromb Res. 2006; 118:275–280.244. Ciciliano JC, Sakurai Y, Myers DR, Fay ME, Hechler B, Meeks S, et al. Resolving the multifaceted mechanisms of the ferric chloride thrombosis model using an interdisciplinary microfluidic approach. Blood. 2015; 126:817–824.
Article245. Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990; 60:269–280.
Article246. Karatas H, Erdener SE, Gursoy-Ozdemir Y, Gurer G, Soylemezoglu F, Dunn AK, et al. Thrombotic distal middle cerebral artery occlusion produced by topical FeCl(3) application: a novel model suitable for intravital microscopy and thrombolysis studies. J Cereb Blood Flow Metab. 2011; 31:1452–1460.
Article247. Kim DE, Kim JY, Sun IC, Schellingerhout D, Lee SK, Ahn CH, et al. Hyperacute direct thrombus imaging using computed tomography and gold nanoparticles. Ann Neurol. 2013; 73:617–625.
Article248. Dietrich WD, Ginsberg MD, Busto R, Watson BD. Photochemically induced cortical infarction in the rat. 1. Time course of hemodynamic consequences. J Cereb Blood Flow Metab. 1986; 6:184–194.
Article249. Hilger T, Blunk JA, Hoehn M, Mies G, Wester P. Characterization of a novel chronic photothrombotic ring stroke model in rats by magnetic resonance imaging, biochemical imaging, and histology. J Cereb Blood Flow Metab. 2004; 24:789–797.
Article250. Hu X, Wester P, Brannstrom T, Watson BD, Gu W. Progressive and reproducible focal cortical ischemia with or without late spontaneous reperfusion generated by a ring-shaped, laser-driven photothrombotic lesion in rats. Brain Res Brain Res Protoc. 2001; 7:76–85.
Article251. Kim HS, Kim D, Kim RG, Kim JM, Chung E, Neto PR, et al. A rat model of photothrombotic capsular infarct with a marked motor deficit: a behavioral, histologic, and microPET study. J Cereb Blood Flow Metab. 2014; 34:683–689.
Article252. Kleinschnitz C, Braeuninger S, Pham M, Austinat M, Nölte I, Renné T, et al. Blocking of platelets or intrinsic coagulation pathway-driven thrombosis does not prevent cerebral infarctions induced by photothrombosis. Stroke. 2008; 39:1262–1268.
Article253. Kuroiwa T, Xi G, Hua Y, Nagaraja TN, Fenstermacher JD, Keep RF. Development of a rat model of photothrombotic ischemia and infarction within the caudoputamen. Stroke. 2009; 40:248–253.
Article254. Lee VM, Burdett NG, Carpenter A, Hall LD, Pambakian PS, Patel S, et al. Evolution of photochemically induced focal cerebral ischemia in the rat: magnetic resonance imaging and histology. Stroke. 1996; 27:2110–2118.255. Lu H, Li Y, Yuan L, Li H, Lu X, Tong S. Induction and imaging of photothrombotic stroke in conscious and freely moving rats. J Biomed Opt. 2014; 19:96013.
Article256. Provenzale JM, Jahan R, Naidich TP, Fox AJ. Assessment of the patient with hyperacute stroke: imaging and therapy. Radiology. 2003; 229:347–359.
Article257. Qian C, Li PC, Jiao Y, Yao HH, Chen YC, Yang J, et al. Precise characterization of the penumbra revealed by MRI: a modified photothrombotic stroke model study. PLoS One. 2016; 11:e0153756.
Article258. Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985; 17:497–504.
Article259. Schroeter M, Jander S, Stoll G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: characterization of inflammatory responses. J Neurosci Methods. 2002; 117:43–49.
Article260. Kleinschnitz C, Bendszus M, Frank M, Solymosi L, Toyka KV, Stoll G. In vivo monitoring of macrophage infiltration in experimental ischemic brain lesions by magnetic resonance imaging. J Cereb Blood Flow Metab. 2003; 23:1356–1361.
Article261. Jander S, Schroeter M, Stoll G. Role of NMDA receptor signaling in the regulation of inflammatory gene expression after focal brain ischemia. J Neuroimmunol. 2000; 109:181–187.
Article262. Jolkkonen J, Jokivarsi K, Laitinen T, Grohn O. Subacute hemorrhage and resolution of edema in Rose Bengal stroke model in rats coincides with improved sensorimotor functions. Neurosci Lett. 2007; 428:99–102.
Article263. Moon SK, Alaverdashvili M, Cross AR, Whishaw IQ. Both compensation and recovery of skilled reaching following small photothrombotic stroke to motor cortex in the rat. Exp Neurol. 2009; 218:145–153.
Article264. Pevsner PH, Eichenbaum JW, Miller DC, Pivawer G, Eichenbaum KD, Stern A, et al. A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation. J Pharmacol Toxicol Methods. 2001; 45:227–233.
Article265. Pierpaoli C, Righini A, Linfante I, Tao-Cheng JH, Alger JR, Di Chiro G. Histopathologic correlates of abnormal water diffusion in cerebral ischemia: diffusion-weighted MR imaging and light and electron microscopic study. Radiology. 1993; 189:439–448.
Article266. Schroeter M, Franke C, Stoll G, Hoehn M. Dynamic changes of magnetic resonance imaging abnormalities in relation to inflammation and glial responses after photothrombotic cerebral infarction in the rat brain. Acta Neuropathol. 2001; 101:114–122.
Article267. van Bruggen N, Cullen BM, King MD, Doran M, Williams SR, Gadian DG, et al. T2- and diffusion-weighted magnetic resonance imaging of a focal ischemic lesion in rat brain. Stroke. 1992; 23:576–582.
Article268. Harrison TC, Silasi G, Boyd JD, Murphy TH. Displacement of sensory maps and disorganization of motor cortex after targeted stroke in mice. Stroke. 2013; 44:2300–2306.
Article269. Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, et al. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci. 2013; 16:55–63.
Article270. Sun YY, Kuo YM, Chen HR, Short-Miller JC, Smucker MR, Kuan CY. A murine photothrombotic stroke model with an increased fibrin content and improved responses to tPA-lytic treatment. Blood Adv. 2020; 4:1222–1231.
Article271. Miao P, Lu H, Liu Q, Li Y, Tong S. Laser speckle contrast imaging of cerebral blood flow in freely moving animals. J Biomed Opt. 2011; 16:090502.
Article272. IST-3 collaborative group, Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012; 379:2352–2363.273. Coutts SB, Berge E, Campbell BC, Muir KW, Parsons MW. Tenecteplase for the treatment of acute ischemic stroke: a review of completed and ongoing randomized controlled trials. Int J Stroke. 2018; 13:885–892.
Article274. Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012; 366:1099–1107.
Article275. Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Rønning OM, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017; 16:781–788.
Article276. Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion–diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009; 8:141–150.
Article277. von Kummer R, Mori E, Truelsen T, Jensen JS, Grønning BA, Fiebach JB, et al. Desmoteplase 3 to 9 hours after major artery occlusion stroke: the DIAS-4 trial (efficacy and safety study of desmoteplase to treat acute ischemic stroke). Stroke. 2016; 47:2880–2887.278. Hill MD, Michel P. Tenecteplase knocking on the door. Stroke. 2018; 49:2276–2277.
Article279. Huang X, MacIsaac R, Thompson JL, Levin B, Buchsbaum R, Haley EC Jr, et al. Tenecteplase versus alteplase in stroke thrombolysis: an individual patient data meta-analysis of randomized controlled trials. Int J Stroke. 2016; 11:534–543.
Article280. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019; 50:2156–2162.
Article281. Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004; 351:2170–2178.
Article282. Salman-Kesner N, Zaltsman MM, Ertracht O, Atar S. In-vitro assessment of the thrombolytic efficacy of therapeutic ultrasound. Thromb Res. 2019; 178:63–68.
Article283. Papadopoulos N, Kyriacou PA, Damianou C. Review of protocols used in ultrasound thrombolysis. J Stroke Cerebrovasc Dis. 2017; 26:2447–2469.
Article284. Bader KB, Bouchoux G, Holland CK. Sonothrombolysis. Adv Exp Med Biol. 2016; 880:339–362.
Article285. Wang Z. An in vitro assay for sonothrombolysis based on the spectrophotometric measurement of clot thickness. J Ultrasound Med. 2017; 36:681–698.
Article286. Amar DN, Epshtein M, Korin N. Endothelial cell activation in an embolic ischemia-reperfusion injury microfluidic model. Micromachines (Basel). 2019; 10:857.287. Parimalam SS, Badilescu S, Sonenberg N, Bhat R, Packirisamy M. Lab-on-a-chip for the development of pro-/anti-angiogenic nanomedicines to treat brain diseases. Int J Mol Sci. 2019; 20:6126.288. Andrzejewska A. Single-cell, high-throughput analysis of cell docking to vessel wall. J Cereb Blood Flow Metab. 2019; 39:2308–2320.
Article289. Griffin MT, Kim D, Ku DN. Shear-induced platelet aggregation: 3D-grayscale microfluidics for repeatable and localized occlusive thrombosis. Biomicrofluidics. 2019; 13:054106.
Article290. Loyau S, Ho-Tin-Noé B, Bourrienne MC, Boulaftali Y, Jandrot-Perrus M. Microfluidic modeling of thrombolysis: effect of antiplatelet and anticoagulant agents on tPA (tissue-type plasminogen activator)-induced fibrinolysis. Arterioscler Thromb Vasc Biol. 2018; 38:2626–2637.291. Costa PF, Albers HJ, Linssen JEA, Middelkamp HHT, van der Hout L, Passier R, et al. Mimicking arterial thrombosis in a 3D-printed microfluidic in vitro vascular model based on computed tomography angiography data. Lab Chip. 2017; 17:2785–2792.
Article292. van Duinen V, Zhu D, Ramakers C, van Zonneveld AJ, Vulto P, Hankemeier T. Perfused 3D angiogenic sprouting in a high-throughput in vitro platform. Angiogenesis. 2019; 22:157–165.
Article293. Kim J, Jang HJ, Schellingerhout D, Lee SK, Kim H, Kim YD, et al. Short-term cessation of dabigatran causes a paradoxical prothrombotic state. Ann Neurol. 2020; Nov. 20. [Epub]. https://doi.org/10.1002/ana.25964.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcranial Doppler Sonography in Acute Ischemic Stroke
- A case of successful treatment of stroke with intracardiac thrombi in a patient with severe ovarian hyperstimulation syndrome
- Review of Stroke Thrombolytics
- Review of Updated Guidelines and Evidence for Antithrombotic Therapy in Acute Ischemic Stroke
- ERRATUM: Author's Name Correction. Evolution of Endovascular Therapy in Acute Stroke: Implications of Device Development