J Bacteriol Virol.
2020 Dec;50(4):218-226. 10.4167/jbv.2020.50.4.218.
A Novel Antibiotic Agent, Cefiderocol, for Multidrug-Resistant Gram-Negative Bacteria
- Affiliations
-
- 1Department of Microbiology and Immunology, School of Medicine and Brain Korea 21 PLUS Program, and Jeju Research Center for Natural Medicine, Jeju National University, Jeju 63243, Republic of Korea
- 2Department of Pharmacy, Jagannath University, Dhaka-1100, Bangladesh
- KMID: 2512141
- DOI: http://doi.org/10.4167/jbv.2020.50.4.218
Abstract
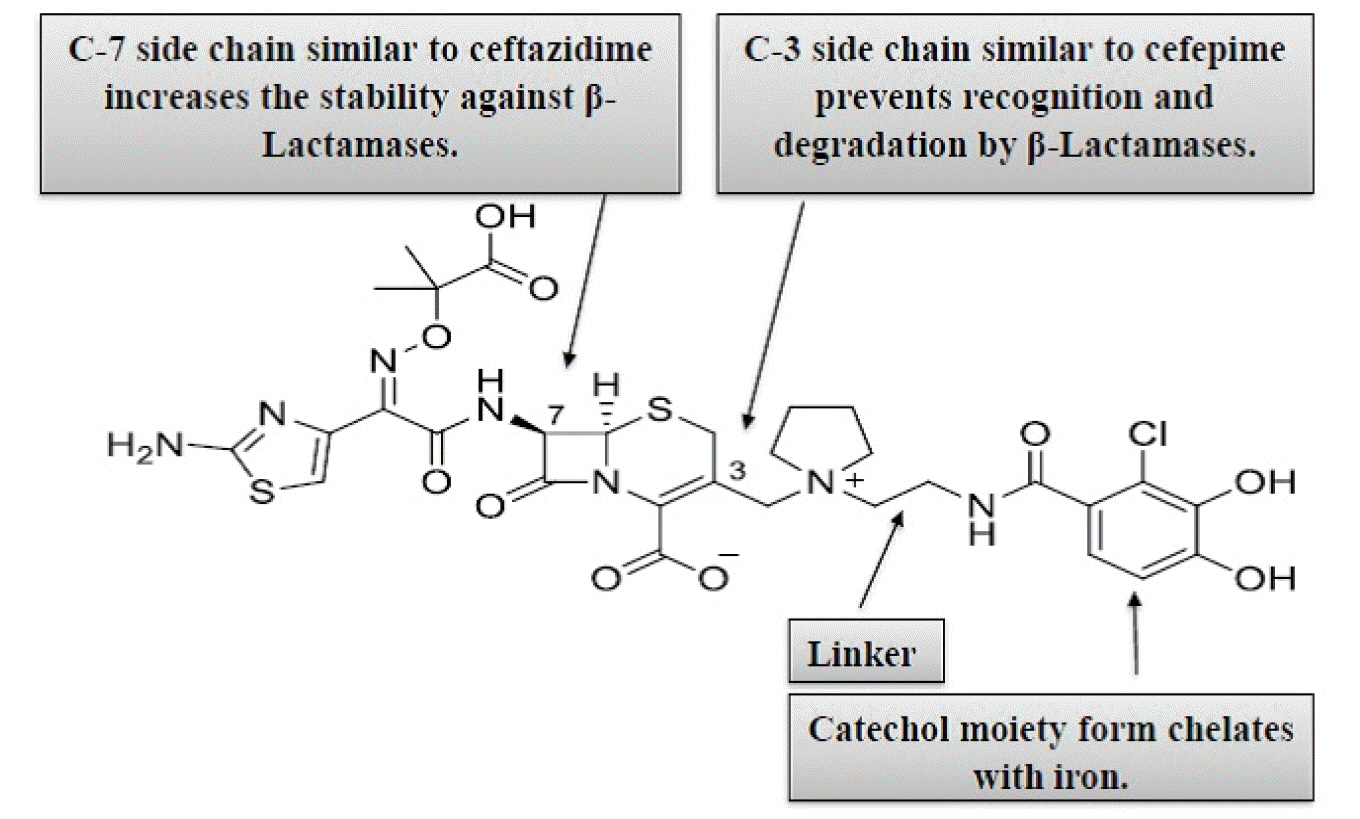
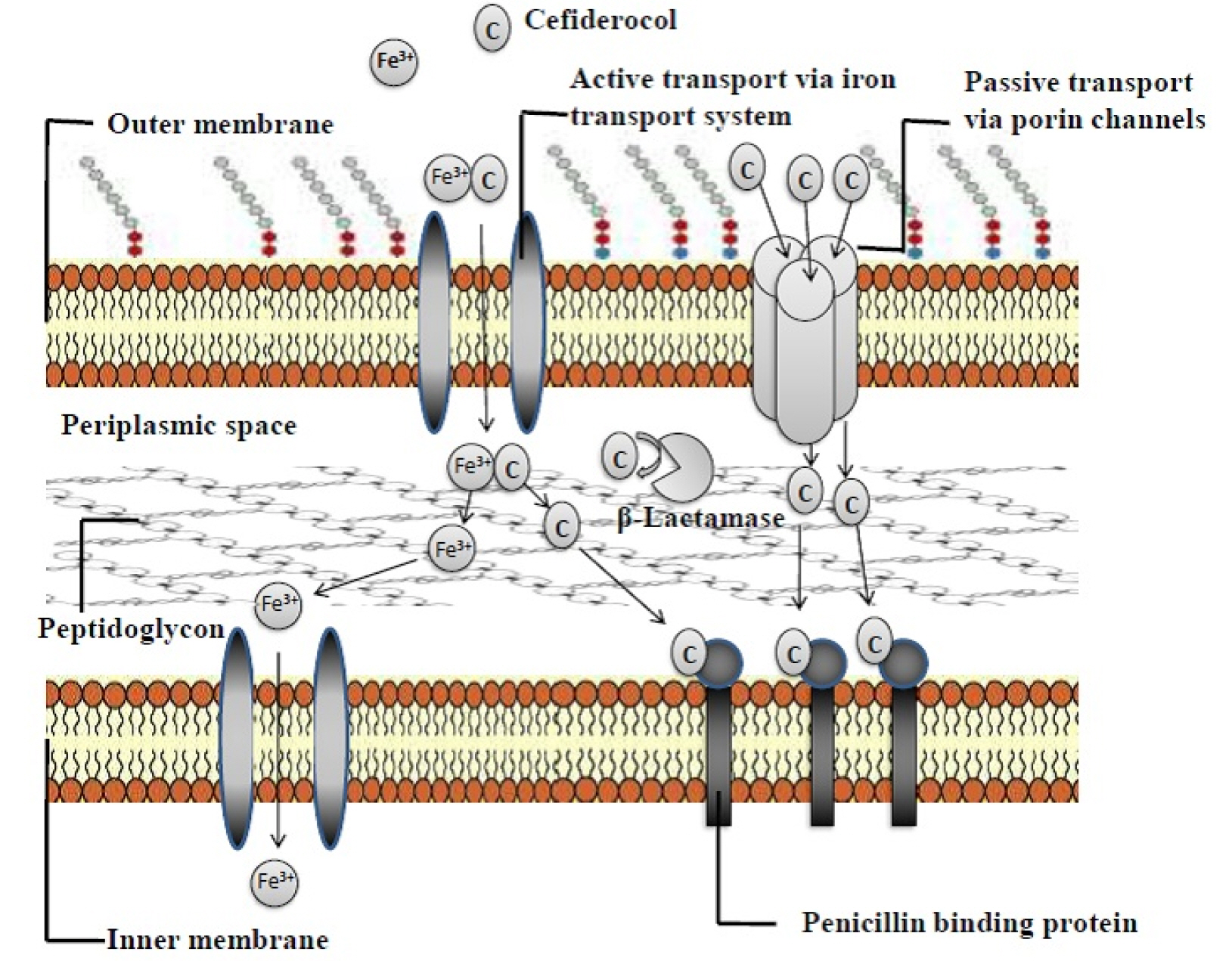
- The threat of antibiotic resistance is an influencing factor in deteriorating public health. Therefore, new antibiotic development is necessary for continued successful treatment of infectious diseases. Cefiderocol is the first licensed injectable siderophore cephalosporin that chemically conjugates a siderophore and cephalosporin. Due to its high stability against various β-lactamases, it is widely used as an effective antibiotic for multidrug-resistant (MDR) gram-negative microorganisms, including Acinetobacter baumannii, Stenotrophomonas maltophilia, Pseudomonas aeruginosa, and Enterobacteriaceae. Cefiderocol blocks microbial cell membrane synthesis. The binding site of cefiderocol is a penicillin-binding protein. Because of its siderophore-like properties, cefiderocol penetrates gram-negative bacterial periplasmic spaces, increasing its stability against β-lactamases. Unlike earlier cephalosporins, the siderophore of the cefiderocol moiety at position C-3 chelates with iron (ferric form) in the host and is then actively transported into the bacterial periplasmic space. This approach is known as a “Trojan horse” and improves cefiderocol stability against efflux pumps as well as porin channel mutations. Modification at the C-3 and C-7 side-chains produces powerful antibacterial properties against MDR gram-negative bacteria. The U.S. Food and Drug Administration (FDA) approved it as a new treatment option for adult patients with complicated urinary tract infection (cUTI) who have limited and no treatment options. Based on these observations, we conclude that cefiderocol is a potent treatment option for prospective bacterial infections. In this review, we summarize the future prospective use of cefiderocol for bacterial infections.
Keyword
Figure
Reference
-
1. Rahman MS, Koh YS. Delafloxacin, a new miracle in antibiotics armamentarium for bacterial infections. J Bacteriol Virol 2019;49:39-43.DOI: 10.4167/jbv.2019.49.1.39.2. Rahman MS, Koh YS. Omadacycline, a magic antibiotics for bacterial infections. J Bacteriol Virol 2018;48:109-12.DOI: 10.4167/jbv.2018.48.3.109.3. Jean SS, Hsueh SC, Lee WS, Hsueh PR. Cefiderocol: a promising antibiotic against multidrug-resistant Gram-negative bacteria. Expert Rev Anti Infect Ther 2019;17:307-9.DOI: 10.1080/14787210.2019.1612240. PMID: 31055983.4. Jean SS, Hsueh PR, SMART Asia-Pacific Group. Distribution of ESBLs, AmpC β-lactamases and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal and urinary tract infections in the Asia-Pacific region during 2008-14: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). J Antimicrob Chemother 2017;72:166-71.DOI: 10.1093/jac/dkw398. PMID: 27703058.5. Jean SS, Lee WS, Lam C, Hsu CW, Chen RJ, Hsueh PR. Carbapenemase-producing Gram-negative bacteria: current epidemics, antimicrobial susceptibility and treatment options. Future Microbiol 2015;10:407-25.DOI: 10.2217/fmb.14.135. PMID: 25812463.6. Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018;18:318-27.7. Falagas ME, Skalidis T, Vardakas KZ, Legakis NJ, Hellenic Cefiderocol Study Group. Activity of cefiderocol (S-649266) against carbapenem-resistant Gram-negative bacteria collected from inpatients in Greek hospitals. J Antimicrob Chemother 2017;72:1704-8.DOI: 10.1093/jac/dkx049. PMID: 28369471.8. Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev 2016;29:321-47.DOI: 10.1128/CMR.00068-15. PMID: 26960938. PMCID: PMC4786888.9. Falagas ME, Mavroudis AD, Vardakas KZ. The antibiotic pipeline for multi-drug resistant gram negative bacteria: what can we expect? Expert Rev Anti Infect Ther 2016;14:747-63.DOI: 10.1080/14787210.2016.1204911. PMID: 27400643.10. Choi JJ, McCarthy MW. Cefiderocol: a novel siderophore cephalosporin. Expert Opin Investig Drugs 2018;27:193-7.DOI: 10.1080/13543784.2018.1426745. PMID: 29318906.11. Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P. Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res Int 2016;23:3984-99.DOI: 10.1007/s11356-015-4294-0. PMID: 25758420.12. Möllmann U, Heinisch L, Bauernfeind A, Köhler T, Ankel-Fuchs D. Siderophores as drug delivery agents: application of the "Trojan Horse" strategy. BioMetals 2009;22:615-24.DOI: 10.1007/s10534-009-9219-2. PMID: 19214755.13. Górska A, Sloderbach A, Marszałł MP. Siderophore-drug complexes: potential medicinal applications of the ’Trojan horse’ strategy. Trends Pharmacol Sci 2014;35:442-9.DOI: 10.1016/j.tips.2014.06.007. PMID: 25108321.14. Page MGP, Dantier C, Desarbre E. In Vitro Properties of BAL30072, a Novel Siderophore Sulfactam with Activity against Multiresistant Gram-Negative Bacilli. Antimicrob Agents Chemother 2010;54:2291-2302.DOI: 10.1128/AAC.01525-09. PMID: 20308379. PMCID: PMC2876421.15. Foley TL, Simeonov A. Targeting iron assimilation to develop new antibacterials. Expert Opin Drug Discov 2012;7:831-47.DOI: 10.1517/17460441.2012.708335. PMID: 22812521. PMCID: PMC3434712.16. Sato T, Yamawaki K. Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Clin Infect Dis 2019;69:S538-43.DOI: 10.1093/cid/ciz826. PMID: 31724047. PMCID: PMC6853759.17. El-Lababidi RM, Rizk JG. Cefiderocol: A Siderophore Cephalosporin. Ann Pharmacother 2020;54(12):1215-31.DOI: 10.1177/1060028020929988. PMID: 32522005.18. Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, et al. Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs 2019;79:271-89.DOI: 10.1007/s40265-019-1055-2. PMID: 30712199.19. Kohira N, West J, Ito A, Ito-Horiyama T, Nakamura R, Sato T, et al. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 2015;60:729-34.DOI: 10.1128/AAC.01695-15. PMID: 26574013. PMCID: PMC4750680.20. Ito-Horiyama T, Ishii Y, Ito A, Sato T, Nakamura R, Fukuhara N, et al. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 2016;60:4384-6.DOI: 10.1128/AAC.03098-15. PMID: 27139465. PMCID: PMC4914688.21. Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant enterobacteriaceae: Epidemiology and prevention. Clin Infect Dis 2011;53:60-7.DOI: 10.1093/cid/cir202. PMID: 21653305.22. Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: Past, present, and future. Antimicrob Agents Chemother 2011;55:4943-60.DOI: 10.1128/AAC.00296-11. PMID: 21859938. PMCID: PMC3195018.23. Wright H, Bonomo RA, Paterson DL. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect 2017;23:704-12.DOI: 10.1016/j.cmi.2017.09.001. PMID: 28893690.24. Wu JY, Srinivas P, Pogue JM. Cefiderocol: A Novel Agent for the Management of Multidrug-Resistant Gram-Negative Organisms. Infect Dis Ther 2020;9:17-40.DOI: 10.1007/s40121-020-00286-6. PMID: 32072491. PMCID: PMC7054475.25. Wunderink RG, Wunderink RG, Matsunaga Y, Ari M, Ariyasu M, Echols R, et al. LB4. Efficacy and Safety of Cefiderocol vs. High-Dose Meropenem in Patients with Nosocomial Pneumonia-Results of a Phase 3, Randomized, Multicenter, Double-Blind, Non-Inferiority Study. Open Forum Infect Dis 2019;6:S994.DOI: 10.1093/ofid/ofz415.2487. PMCID: PMC6810733.26. Kufel WD, Steele JM, Riddell SW, Jones Z, Shakeraneh P, Endy TP. Cefiderocol for treatment of an empyema due to extensively drug-resistant Pseudomonas aeruginosa: Clinical observations and susceptibility testing considerations. IDCases 2020;21:e00863.DOI: 10.1016/j.idcr.2020.e00863. PMID: 32577400. PMCID: PMC7300106.27. Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, et al. Siderophore Cephalosporin Cefiderocol Utilizes Ferric Iron Transporter Systems for Antibacterial Activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2016;60:7396-401.DOI: 10.1128/AAC.01405-16. PMID: 27736756. PMCID: PMC5119021.28. Dunn GL. Ceftizoxime and other third-generation cephalosporins: structure-activity relationships. J Antimicrob Chemother 1982;10:1-10.DOI: 10.1093/jac/10.suppl_C.1. PMID: 6295993.29. Neu HC. β-Lactam Antibiotics: Structural Relationships Affecting in Vitro Activity and Pharmacologic Properties. Rev Infect Dis 1986;8:S237-59.DOI: 10.1093/clinids/8.Supplement_3.S237. PMID: 3529320.30. Aoki T, Yoshizawa H, Yamawaki K, Yokoo K, Sato J, Hisakawa S, et al. Cefiderocol (S-649266), A new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: Structure activity relationship. Eur J Med Chem 2018;155:847-68.DOI: 10.1016/j.ejmech.2018.06.014. PMID: 29960205.31. Ito A, Nishikawa T, Yamano Y, Matsumoto S, Yoshizawa H, Sato T, et al. Siderophore Cephalosporin Cefiderocol Utilizes Ferric Iron Transporter Systems for Antibacterial Activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2016;60:7396-401.DOI: 10.1128/AAC.01405-16. PMID: 27736756. PMCID: PMC5119021.32. Grassi GG, Grassi C. Cefepime: overview of activity in vitro and in vivo. J Antimicrob Chemother 1993;32:87-94.DOI: 10.1093/jac/32.suppl_B.87. PMID: 8150771.33. Ito A, Sato T, Ota M, Takemura M, Nishikawa T, Toba S, et al. In Vitro Antibacterial Properties of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacteria. Antimicrob Agents Chemother 2017;62:e01454-17.DOI: 10.1128/AAC.01454-17. PMID: 29061741. PMCID: PMC5740388.34. Alegria W. Cefiderocol (Fetroja). Infect Dis Alert 2020;39:1-4.35. Yamano Y. In Vitro Activity of Cefiderocol Against a Broad Range of Clinically Important Gram-negative Bacteria. Clin Infect Dis 2019;69:S544-51.DOI: 10.1093/cid/ciz827. PMID: 31724049. PMCID: PMC6853761.36. Matsumoto S, Singley CM, Hoover J, Nakamura R, Echols R, Rittenhouse S, et al. Efficacy of Cefiderocol against Carbapenem-Resistant Gram-Negative Bacilli in Immunocompetent-Rat Respiratory Tract Infection Models Recreating Human Plasma Pharmacokinetics. Antimicrob Agents Chemother 2017;61:e00700-17.DOI: 10.1128/AAC.00700-17. PMID: 28630178. PMCID: PMC5571323.37. Nakamura R, Ito-Horiyama T, Takemura M, Toba S, Matsumoto S, Ikehara T, et al. In Vivo Pharmacodynamic Study of Cefiderocol, a Novel Parenteral Siderophore Cephalosporin, in Murine Thigh and Lung Infection Models. Antimicrob Agents Chemother 2019;63:e02031-18.DOI: 10.1128/AAC.02031-18. PMID: 31262762. PMCID: PMC6709502.38. Saisho Y, Katsube T, White S, Fukase H, Shimada J. Pharmacokinetics, Safety, and Tolerability of Cefiderocol, a Novel Siderophore Cephalosporin for Gram-Negative Bacteria, in Healthy Subjects. Antimicrob Agents Chemother 2018;62:e02163-17.DOI: 10.1128/AAC.02163-17. PMID: 29311072. PMCID: PMC5826143.39. Katsube T, Echols R, Arjona Ferreira JC, Krenz HK, Berg JK, Galloway C. Cefiderocol, a Siderophore Cephalosporin for Gram-Negative Bacterial Infections: Pharmacokinetics and Safety in Subjects With Renal Impairment. J Clin Pharmacol 2017;57:584-91.DOI: 10.1002/jcph.841. PMID: 27874971. PMCID: PMC5412848.40. Kawaguchi N, Katsube T, Echols R, Wajima T. Population Pharmacokinetic Analysis of Cefiderocol, a Parenteral Siderophore Cephalosporin, in Healthy Subjects, Subjects with Various Degrees of Renal Function, and Patients with Complicated Urinary Tract Infection or Acute Uncomplicated Pyelonephritis. Antimicrob Agents Chemother 2018;62:e01391-17.DOI: 10.1128/AAC.01391-17. PMID: 29038272. PMCID: PMC5786804.41. Katsube T, Wajima T, Ishibashi T, Arjona Ferreira JC, Echols R. Pharmacokinetic/Pharmacodynamic Modeling and Simulation of Cefiderocol, a Parenteral Siderophore Cephalosporin, for Dose Adjustment Based on Renal Function. Antimicrob Agents Chemother 2016;61:e01381-16.DOI: 10.1128/AAC.01381-16. PMID: 27795374. PMCID: PMC5192099.42. Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Arjona Ferreira JC, Ariyasu M, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2018;18:1319-28.DOI: 10.1016/S1473-3099(18)30554-1.43. Katsube T, Miyazaki S, Narukawa Y, Hernandez-Illas M, Wajima T. Drug-drug interaction of cefiderocol, a siderophore cephalosporin, via human drug transporters. Eur J Clin Pharmacol 2018;74:931-8.DOI: 10.1007/s00228-018-2458-9. PMID: 29627897.44. McCarthy MW. Cefiderocol to treat complicated urinary tract infection. Drugs Today (Barc) 2020;56:177-84.DOI: 10.1358/dot.2020.56.3.3118466. PMID: 32282864.45. Li XZ, Plésiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 2015;28:337-418.DOI: 10.1128/CMR.00117-14. PMID: 25788514. PMCID: PMC4402952.46. Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med 2012;27:128-42.DOI: 10.3904/kjim.2012.27.2.128. PMID: 22707882. PMCID: PMC3372794.47. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and Europe, Including Carbapenem-Nonsusceptible Isolates (SIDERO-WT-2014 Stud). Antimicrob Agents Chemother 2017;61:e00093-17.DOI: 10.1128/AAC.00093-17. PMID: 28630181. PMCID: PMC5571285.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multidrug-resistant bacteria: a national challenge requiring urgent addressal
- Antibiotics for multidrug-resistant gram-negative bacteria
- Multidrug-Resistant Gram-Positive Bacterial Infections
- Strategies to combat Gram-negative bacterial resistance to conventional antibacterial drugs: a review
- Therapeutic strategy for the management of multidrug-resistant gram-negative bacterial infections