J Dent Rehabil Appl Sci.
2020 Sep;36(3):145-157. 10.14368/jdras.2020.36.3.145.
Analysis of cell survival genes in human gingival fibroblasts after sequential release of trichloroacetic acid and epidermal growth factor using the nano-controlled release system
- Affiliations
-
- 1Department of Biomaterials & Prosthodontics, Kyung Hee University Hospital at Gangdong, School of Dentistry, Kyung Hee University, Seoul, Republic of Korea
- KMID: 2512109
- DOI: http://doi.org/10.14368/jdras.2020.36.3.145
Abstract
- Purpose
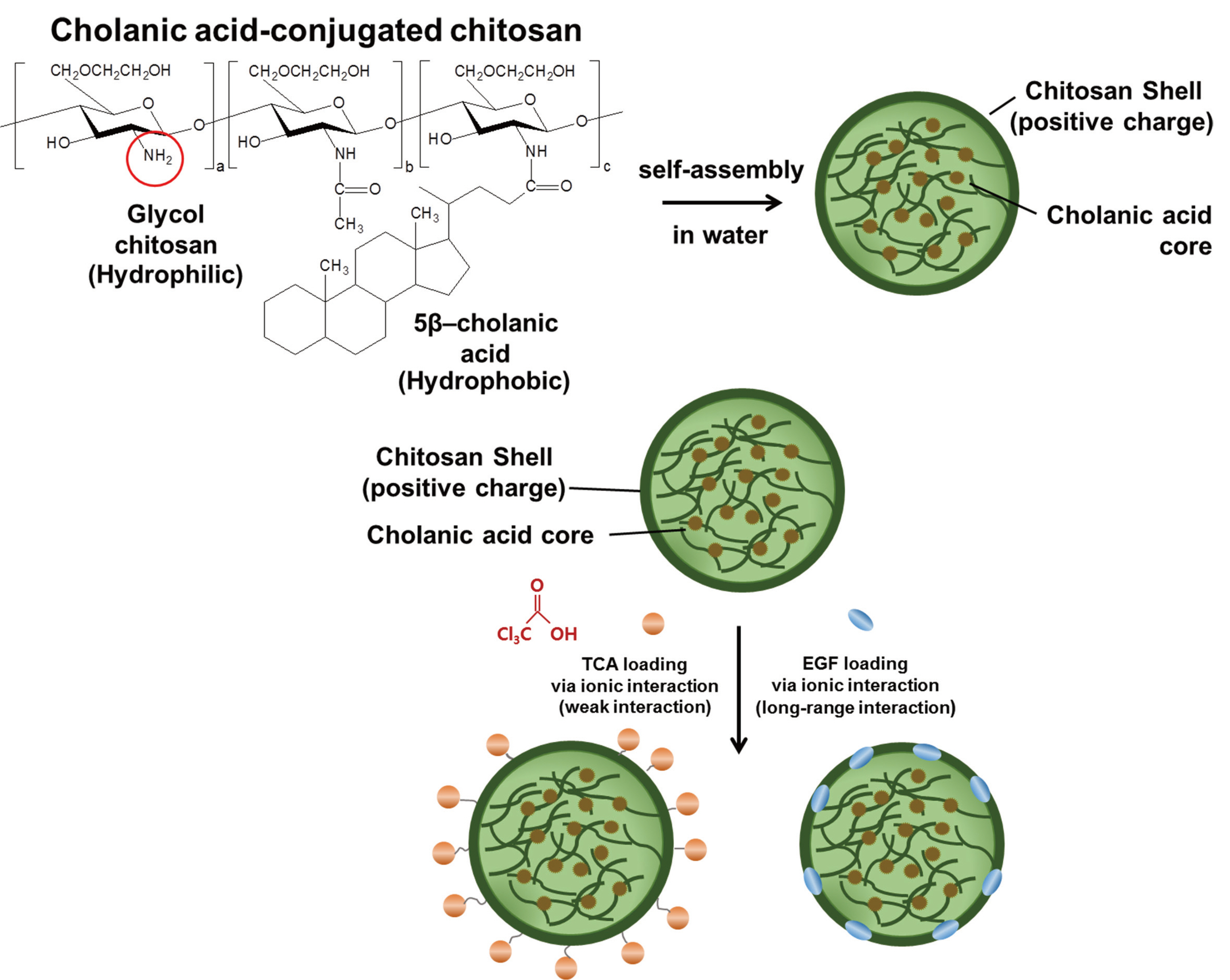
This study was to determine the possible effects of trichloroacetic acid (TCA) and epidermal growth factor (EGF) through cell survival genes of the PI3K-AKT signaling pathway when applying an hydrophobically modified glycol chitosan (HGC)-based nanocontrolled release system to human gingival fibroblasts in oral soft tissue regeneration.
Materials and Methods
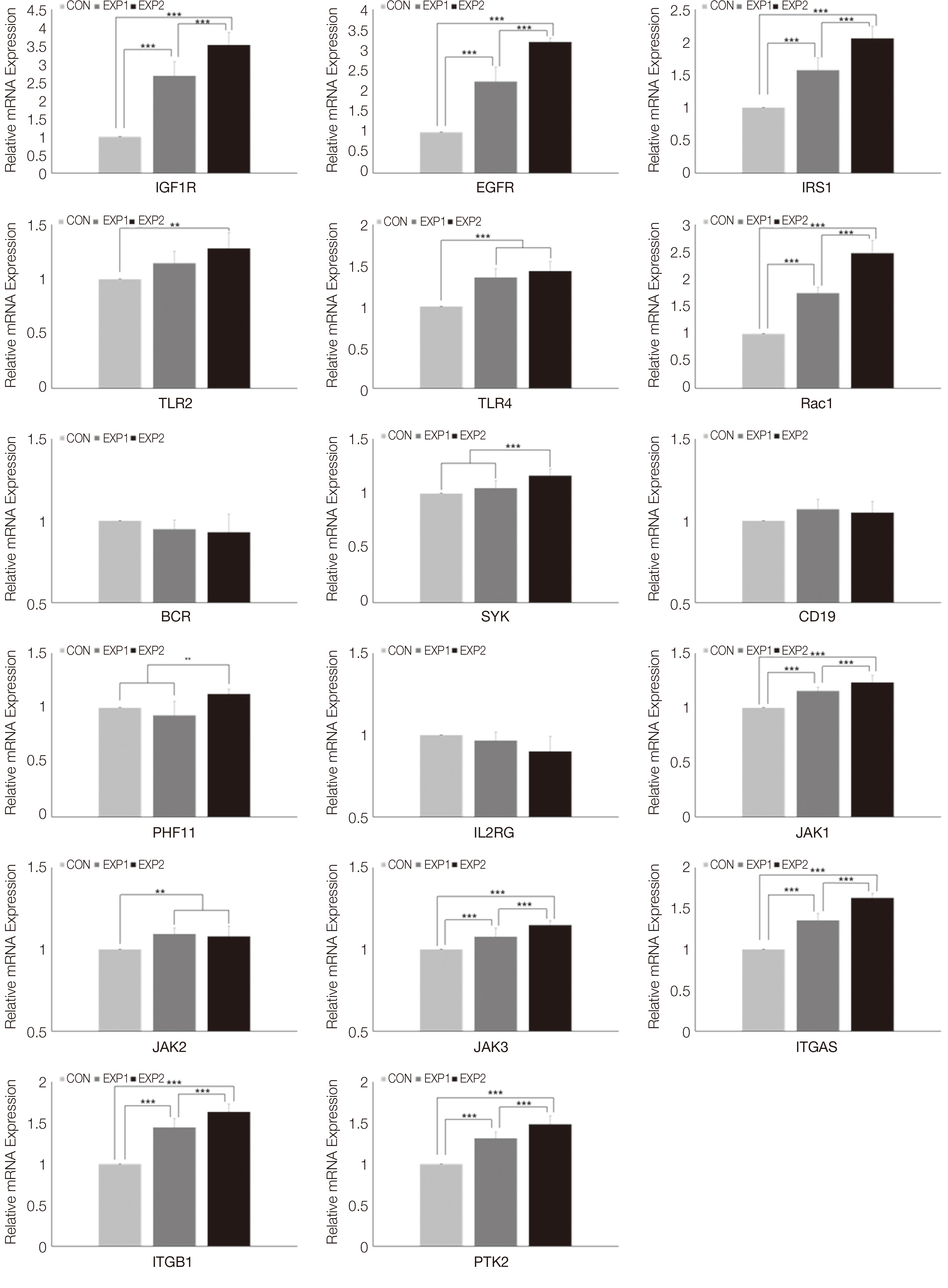
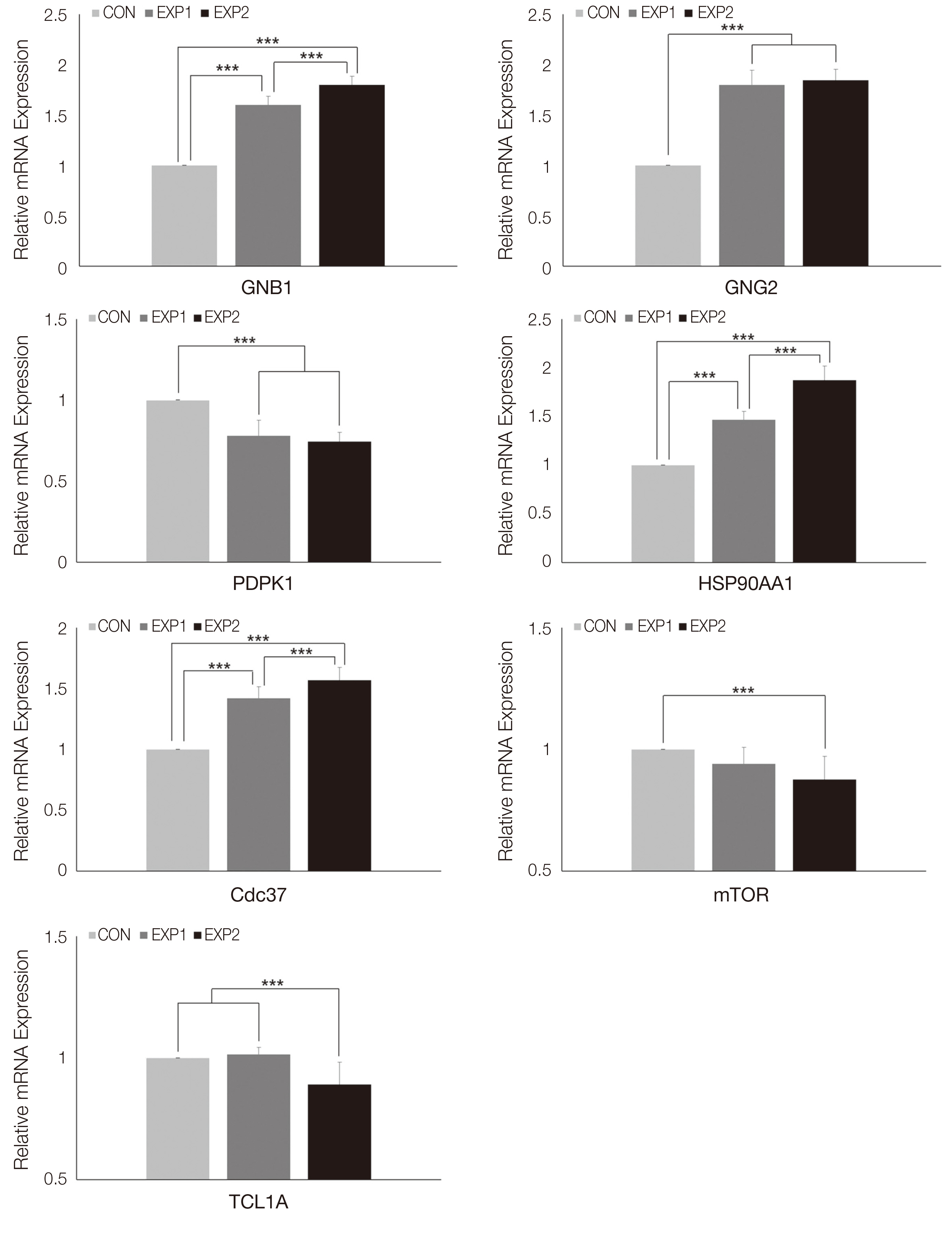
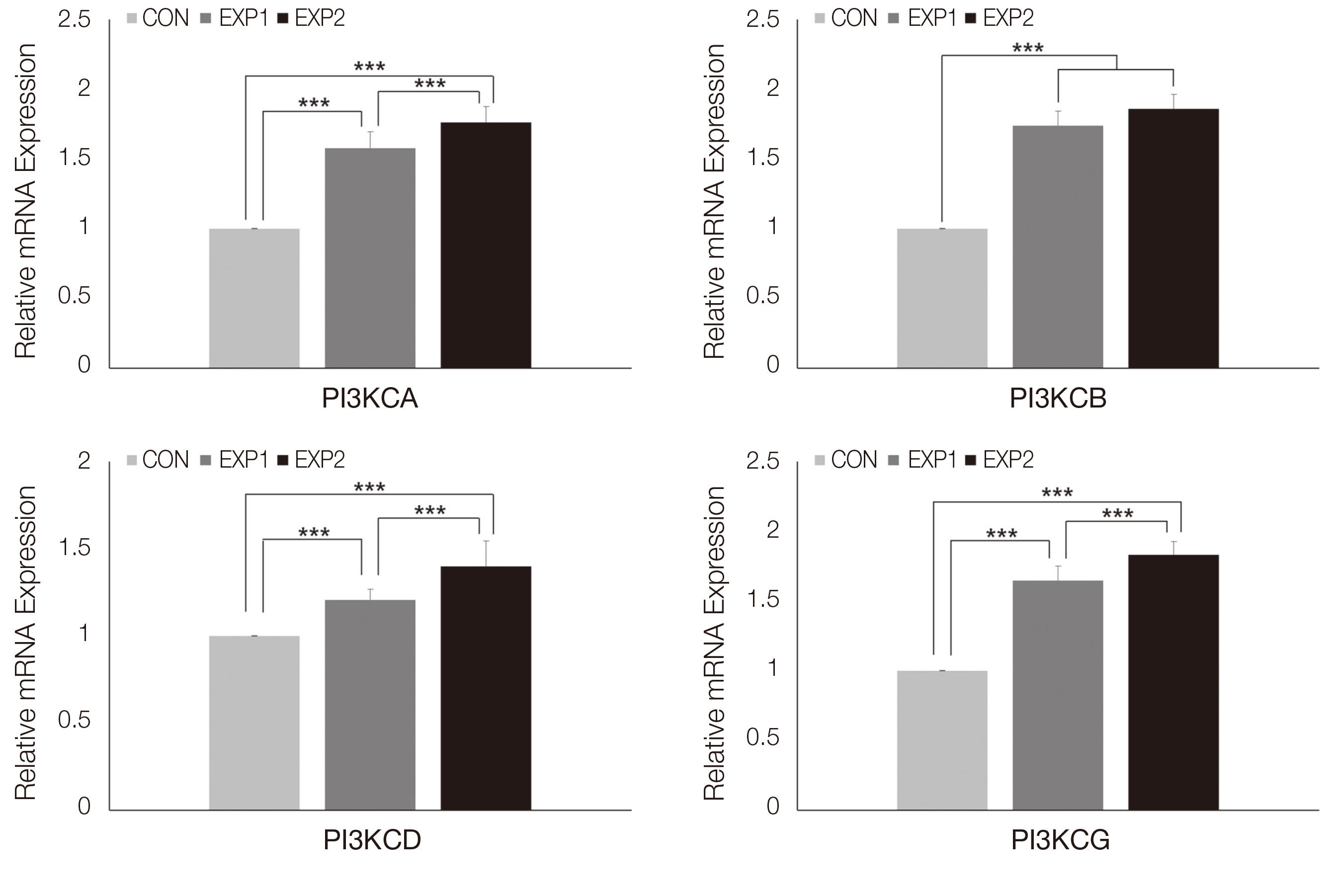
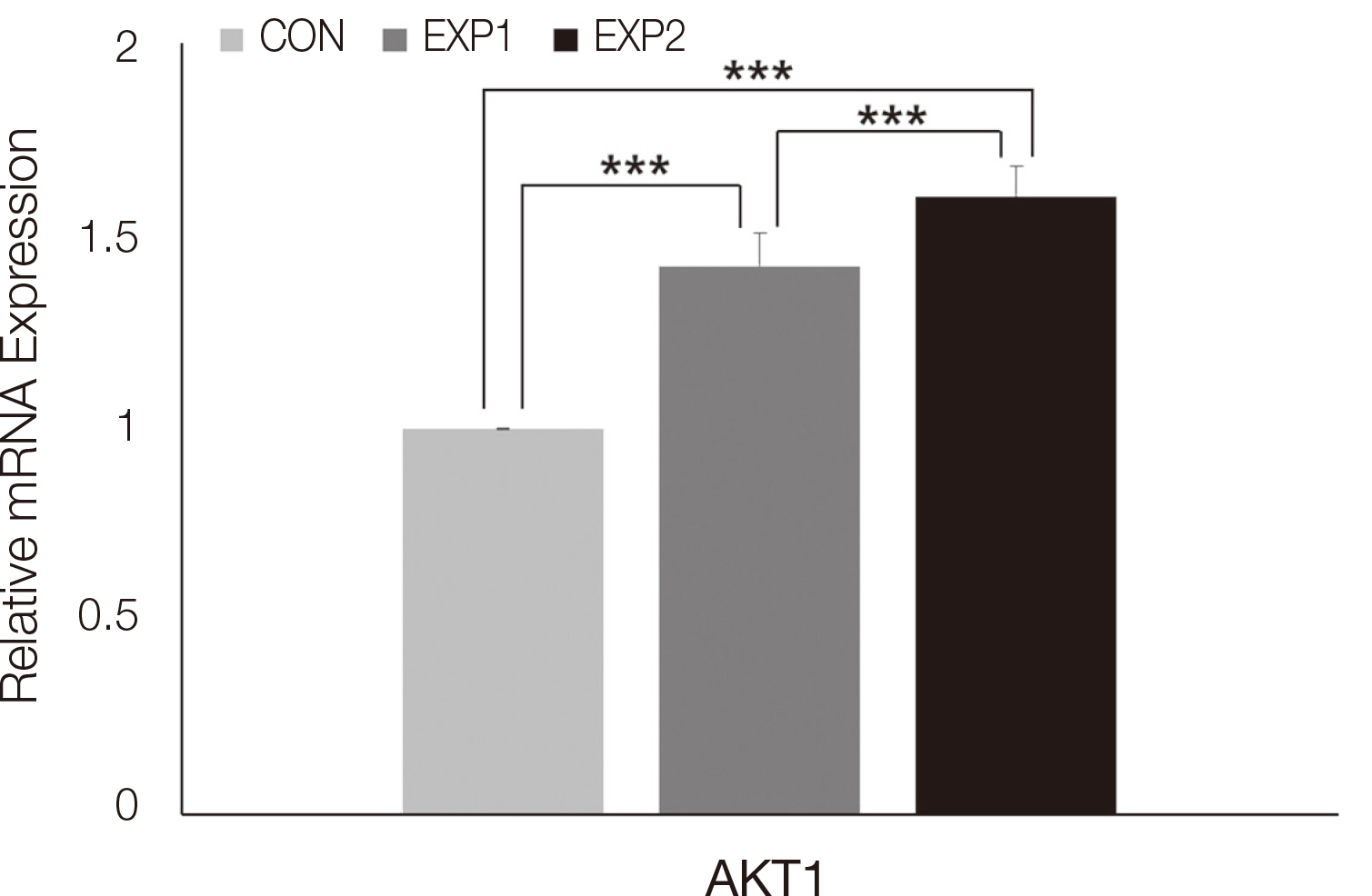
An HGC-based nano-controlled release system was produced, followed by the loading of TCA and EGF. The group was divided into control (CON), TCA-loaded nano-controlled release system (EXP1), and the TCA- and EGF- individually loaded nano-controlled release system (EXP2).A total for 29 genes related to the PI3K-AKT signaling pathway were analyzed after 48h of culture in human gingival fibroblasts. Real-time PCR, 1- way ANOVA and multiple regression analysis were performed.
Results
Cell survival genes were significantly upregulated in EXP1 and EXP2. From multiple regression analysis, ITGB1 was determined to be the most influential factor for AKT1 expression.
Conclusion
The application of TCA and EGF through the HGC-based nano-controlled release system can up-regulate the cell survival pathway.
Keyword
Figure
Reference
-
References
1. Yu JS, Cui W. 2016; Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 143:3050–60. DOI: 10.1242/dev.137075. PMID: 27578176.2. Osaki M, Oshimura M, Ito H. 2004; The PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 9:667–76. DOI: 10.1023/B:APPT.0000045801.15585.dd. PMID: 15505410.3. Manning BD, Cantley LC. 2007; AKT/PKB Signaling: Navigating Downstream. Cell. 129:1261–74. DOI: 10.1016/j.cell.2007.06.009. PMID: 17604717. PMCID: PMC2756685.4. Cheung M, Testa JR. 2013; Diverse mechanisms of AKT pathway activation in human malignancy. Curr Cancer Drug Targets. 13:234–44. DOI: 10.2174/1568009611313030002. PMID: 23297823. PMCID: PMC3678724.5. Romano G. 2013; The role of the dysfunctional akt-related pathway in cancer: Establishment and maintenance of a malignant cell phenotype, resistance to therapy, and future strategies for drug development. Scientifica (Cairo). 2013:317186. DOI: 10.1155/2013/317186. PMID: 24381788. PMCID: PMC3870877.6. Virtakoivu R, Pellinen T, Rantala JK, Perälä M, Ivaska J. 2012; Distinct roles of AKT isoforms in regulating β1-integrin activity, migration, and invasion in prostate cancer. Mol Biol Cell. 23:3357–69. DOI: 10.1091/mbc.E12-03-0213. PMID: 22809628. PMCID: PMC3431929.7. Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, Schultz G, Nürnberg B. 2003; Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J Cell Biol. 160:89–99.8. Stephens L, Eguinoa A, Corey S, Jackson T, Hawkins PT. 1993; Receptor stimulated accumulation of phosphatidylinositol (3,4,5)-trisphosphate by Gprotein mediated pathways in human myeloid derived cells. EMBO J. 12:2265–73. PMCID: PMC413455. PMID: 8389691.9. Reiske HR, Zhao J, Han DC, Cooper LA, Guan JL. 2000; Analysis of FAK-associated signaling pathways in the regulation of cell cycle progression. FEBS Lett. 486:275–80. DOI: 10.1016/s0014-5793(00)02295-x. PMID: 11119718.10. Hlobilková A, Knillová J, Bártek J, Lukás J, Kolár Z. 2003; The mechanism of action of the tumour suppressor gene PTEN. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 147:19–25. PMID: 15034601.11. Sly LM, Rauh MJ, Kalesnikoff J, Büchse T, Krystal G. 2003; SHIP, SHIP2, and PTEN activities are regulated in vivo by modulation of their protein levels: SHIP is up-regulated in macrophages and mast cells by lipopolysaccharide. Exp Hematol. 31:1170–81. DOI: 10.1016/j.exphem.2003.09.011. PMID: 14662322.12. Kalesnikoff J, Sly LM, Hughes MR, Büchse T, Rauh MJ, Cao LP, Lam V, Mui A, Huber M, Krystal G. 2003; The role of SHIP in cytokine-induced signaling. Rev Physiol Biochem Pharmacol. 149:87–103. DOI: 10.1016/j.exphem.2003.09.011. PMID: 12692707.13. Lee KH, Ben Amara H, Lee SC, Leesungbok R, Chung MA, Koo KT, Lee SW. 2019; Chemical regeneration of wound defects: relevance to the canine palatal mucosa and cell cycle up-regulation in human gingival fibroblasts. Tissue Eng Regen Med. 16:675–84. DOI: 10.1007/s13770-019-00227-6. PMID: 31824829. PMCID: PMC6879698.14. Park KM, Lee HJ, Koo KT, Ben Amara HB, Leesungbok R, Noh K, Lee SC, Lee SW. 2020; Oral soft tissue regeneration using the nano controlled system inducing sequential release of trichloroacetic acid and epidermal growth factor. Tissue Eng Regen Med. 17:91–103. DOI: 10.1007/s13770-019-00232-9. PMCID: PMC6992799. PMID: 31970697.15. Song G, Ouyang G, Bao S. 2005; The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 9:59–71. DOI: 10.1111/j.1582-4934.2005.tb00337.x. PMCID: PMC6992799. PMID: 15784165.16. Lateef Z, Wise LM. 2018; Exploitation of receptor tyrosine kinases by viral-encoded growth factors. Growth factors. 36:118–40. DOI: 10.1080/08977194.2018.1520229. PMID: 31084274.17. White MF, Kahn CR. 1994; The insulin signaling system. J Biol Chem. 269:1–4. PMID: 8276779.18. Kawai T, Akira S. 2010; The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 11:373–84. DOI: 10.1038/ni.1863. PMID: 20404851.19. Yokozeki T, Adler K, Lankar D, Bonnerot C. 2003; B cell receptor-mediated Syk-independent activation of phosphatidylinositol 3-kinase, Ras, and mitogenactivated protein kinase pathways. J Immunol. 171:1328–35. DOI: 10.4049/jimmunol.171.3.1328. PMID: 12874222.20. Lankester AC, Rood PM, van Schijndel GM, Hooibrink B, Verhoeven AJ, van Lier RA. 1996; Alteration of B-cell antigen receptor signaling by CD19 coligation. A study with bispecific antibodies. J Biol Chem. 271:22326–30. DOI: 10.1074/jbc.271.37.22326. PMID: 8798392.21. Kurosaki T. 2002; Regulation of B-cell signal transduction by adaptor proteins. Nat Rev Immunol. 2:354–63. DOI: 10.1038/nri801. PMID: 12033741.22. Yamazaki T, Takeda K, Gotoh K, Takeshima H, Akira S, Kurosaki T. 2002; Essential immunoregulatory role for BCAP in B cell development and function. J Exp Med. 195:535–45. DOI: 10.1084/jem.20011751. PMCID: PMC6992799. PMID: 11877477.23. Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, et al. 1994; Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 266:1042–5. DOI: 10.1126/science.7973658. PMID: 7973658.24. Foster FM, Traer CJ, Abraham SM, Fry MJ. 2003; The phosphoinositide (PI) 3-kinase family. J Cell Sci. 116(Pt 15):3037–40. DOI: 10.1242/jcs.00609. PMID: 12829733.25. Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. 1988; Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 4:487–525. DOI: 10.1146/annurev.cb.04.110188.002415. PMID: 3058164.26. Patel J, Channon KM, McNeill E. 2013; The downstream regulation of chemokine receptor signalling: implications for atherosclerosis. Mediators Inflamm. 2013:459520. DOI: 10.1155/2013/459520. PMCID: PMC6992799. PMID: 23690662.27. Matheny RW Jr, Adamo ML. 2009; Current perspectives on Akt Akt-ivation and Akt-ions. Exp Biol Med (Maywood). 234:1264–70. DOI: 10.3181/0904-MR-138. PMID: 19596822.28. Laine J, Künstle G, Obata T, Sha M, Noguchi M. 2000; The protooncogene TCL1 is an Akt kinase coactivator. Mol Cell. 6:395–407. DOI: 10.1016/s1097-2765(00)00039-3. PMID: 10983986.29. Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. 2002; Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 277:39858–66. DOI: 10.1074/jbc.M206322200. PMID: 12176997.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect and mechanism of chitosan-based nano-controlled release system on the promotion of cell cycle progression gene expression
- Analysis of the effect of trichloroacetic acid and epidermal growth factor release on cytoskeleton gene expression using the nano-controlled releasing system
- Oral Soft Tissue Regeneration Using Nano Controlled System Inducing Sequential Release of Trichloroacetic Acid and Epidermal Growth Factor
- Transforming growth factor-beta promoted vascular endothelial growth factor release by human lung fibroblasts
- The Effects of Injectable Platelet-Rich Fibrin and AdvancedPlatelet Rich Fibrin on Gingival Fibroblast Cell Vitality, Proliferation, Differentiation