Efficacy and safety of vedolizumab in Crohn’s disease in patients from Asian countries in the GEMINI 2 study
- Affiliations
-
- 1Asian Institute of Gastroenterology, Hyderabad, India
- 2Singapore General Hospital, Singapore
- 3University Malaya Medical Centre, Kuala Lumpur, Malaysia
- 4Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 5University of Ulsan College of Medicine, Seoul, Korea
- 6Takeda Pharmaceutical International AG, Singapore
- 7Takeda Pharmaceutical International AG, Zurich, Switzerland
- KMID: 2512104
- DOI: http://doi.org/10.5217/ir.2019.09160
Abstract
- Background/Aims
The efficacy and safety of vedolizumab in moderate-to-severely active Crohn’s disease (CD) were demonstrated in the GEMINI 2 study (NCT00783692). This post-hoc exploratory analysis aimed to assess the efficacy and safety of vedolizumab in the subgroup of patients from Asian countries.
Methods
During the induction phase (doses at day 1, 15), clinical remission, enhanced clinical response, and change in C-reactive protein at 6 weeks; during the maintenance phase, clinical remission, enhanced clinical response, glucocorticoid-free remission and durable clinical remission at 52 weeks, were the efficacy outcomes of interest. Efficacy and safety of vedolizumab compared to placebo were assessed in Asian countries (Hong Kong, India, Malaysia, Singapore, South Korea, and Taiwan) using descriptive analyses.
Results
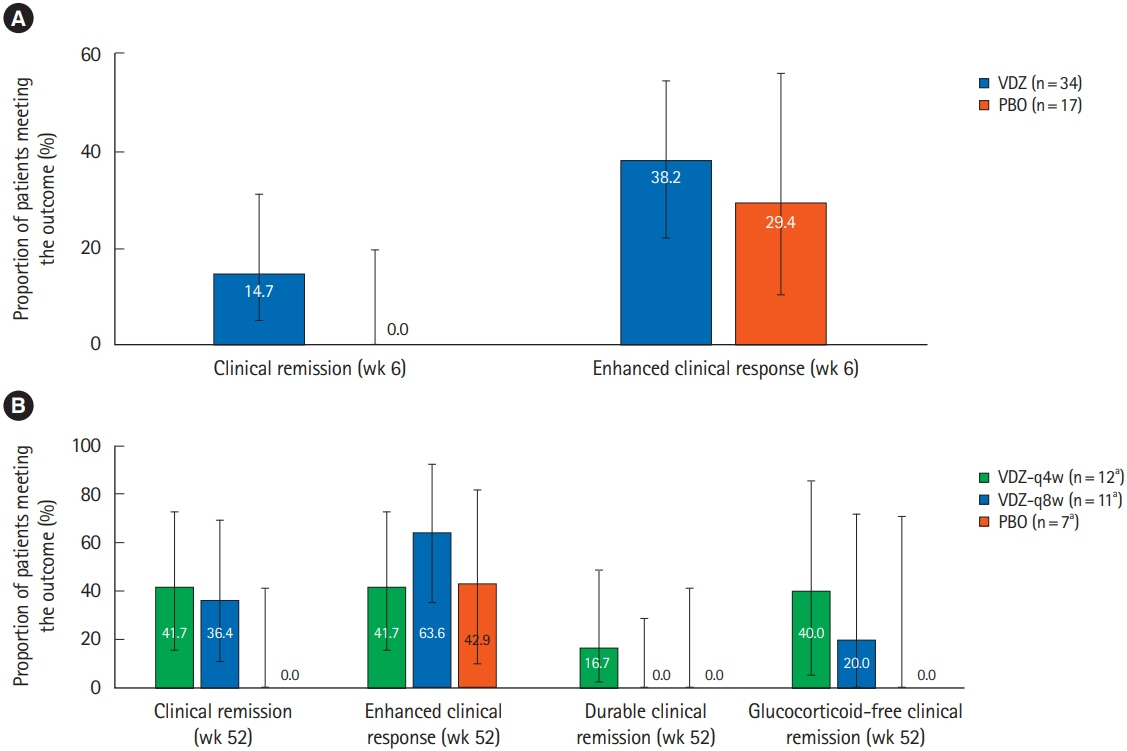
During the induction phase, in Asian countries (n = 51), 14.7% of the vedolizumab-treated patients achieved clinical remission at week 6 compared to none with placebo (difference, 14.7%; 95% confidence interval, 15.8%–43.5%). In non-Asian countries (n = 317), the remission rate at week 6 with vedolizumab was 14.5%. During maintenance, in Asian countries, clinical remission rates at 52 weeks with vedolizumab administered every 4 weeks, vedolizumab administered every 8 weeks and placebo were 41.7%, 36.4%, and 0%, respectively; while enhanced clinical response rates were 41.7%, 63.6%, and 42.9%, respectively. During induction, 39.7% of patients with vedolizumab experienced an adverse event compared to 58.8% of patients with placebo, and vedolizumab was generally well-tolerated.
Conclusions
This post-hoc analysis demonstrates the treatment effect and safety of vedolizumab in moderateto-severely active CD in patients from Asian countries.
Keyword
Figure
Cited by 4 articles
-
The Risk of Tuberculosis in Patients With Inflammatory Bowel Disease Treated With Vedolizumab or Ustekinumab in Korea
Myeong Geun Choi, Byong Duk Ye, Suk-Kyun Yang, Tae Sun Shim, Kyung-Wook Jo, Sang Hyoung Park
J Korean Med Sci. 2022;37(14):e107. doi: 10.3346/jkms.2022.37.e107.Prevention of postoperative recurrence in Crohn’s disease: the never-ending story
Jung-Bin Park, Sang Hyoung Park
Intest Res. 2022;20(3):279-280. doi: 10.5217/ir.2022.00081.Natural history of inflammatory bowel disease: a comparison between the East and the West
Eun Mi Song, Suk-Kyun Yang
Intest Res. 2022;20(4):418-430. doi: 10.5217/ir.2021.00104.Concomitant ankylosing spondylitis can increase the risk of biologics or small molecule therapies to control inflammatory bowel disease
Yu Kyung Jun, Hyuk Yoon, Seong-Joon Koh, A Hyeon Kim, Kwang Woo Kim, Jun Won Park, Hyun Jung Lee, Hyoun Woo Kang, Jong Pil Im, Young Soo Park, Joo Sung Kim
Intest Res. 2023;21(2):244-251. doi: 10.5217/ir.2022.00057.
Reference
-
1. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012; 142:46–54.
Article2. Ng SC, Tsoi KK, Kamm MA, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012; 18:1164–1176.
Article3. Kim ES, Kim WH. Inflammatory bowel disease in Korea: epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010; 4:1–14.
Article4. Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012; 27:1266–1280.
Article5. Ng SC. Epidemiology of inflammatory bowel disease: focus on Asia. Best Pract Res Clin Gastroenterol. 2014; 28:363–372.
Article6. Wang YF, Zhang H, Ouyang Q. Clinical manifestations of inflammatory bowel disease: East and West differences. J Dig Dis. 2007; 8:121–127.
Article7. Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn’s disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008; 14:741–746.
Article8. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.
Article9. Ng SC, Kaplan GG, Tang W, et al. Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am J Gastroenterol. 2019; 114:107–115.
Article10. Ooi CJ, Makharia GK, Hilmi I, et al. Asia Pacific Consensus Statements on Crohn’s disease. Part 1: definition, diagnosis, and epidemiology (Asia Pacific Crohn’s Disease Consensus: part 1). J Gastroenterol Hepatol. 2016; 31:45–55.
Article11. Ooi CJ, Makharia GK, Hilmi I, et al. Asia-Pacific Consensus Statements on Crohn’s disease. Part 2: management. J Gastroenterol Hepatol. 2016; 31:56–68.
Article12. Wong U, Cross RK. Primary and secondary nonresponse to infliximab: mechanisms and countermeasures. Expert Opin Drug Metab Toxicol. 2017; 13:1039–1046.
Article13. U.S. National Library of Medicine: ClinicalTrials.gov. Study of vedolizumab (MLN0002) in patients with moderate to severe Crohn’s disease (GEMINI II) [Internet]. c2014 [cited 2018 Mar 30]. https://clinicaltrials.gov/ct2/show/NCT00783692.14. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013; 369:711–721.
Article15. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol. 2018; 53:1048–1064.
Article16. Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015; 41:1227–1236.
Article17. Ng SC, Hilmi IN, Blake A, et al. Low frequency of opportunistic infections in patients receiving vedolizumab in clinical trials and post-marketing setting. Inflamm Bowel Dis. 2018; 24:2431–2441.
Article18. Amiot A, Grimaud JC, Peyrin-Biroulet L, et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016; 14:1593–1601.19. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017; 66:839–851.
Article20. Cheon JH. Understanding the complications of anti-tumor necrosis factor therapy in East Asian patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2017; 32:769–777.
Article21. Leung WK, Ng SC, Chow DK, et al. Use of biologics for inflammatory bowel disease in Hong Kong: consensus statement. Hong Kong Med J. 2013; 19:61–68.22. Leung WK. Optimization of inflammatory bowel disease cohort studies in Asia. Intest Res. 2015; 13:208–212.
Article23. Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: a phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019; 14:e0212989.
Article24. Watanabe K, Motoya S, Ogata H, et al. Effects of vedolizumab in Japanese patients with Crohn’s disease: a prospective, multicenter, randomized, placebo-controlled phase 3 trial with exploratory analyses. J Gastroenterol. 2020; 55:291–306.
Article25. Gan AT, Chan WP, Ling KL, et al. P634 Real-world data on the efficacy and safety of vedolizumab therapy in patients with inflammatory bowel disease: a retrospective nation-wide cohort study in Singapore. J Crohns Colitis. 2019; 13 Suppl 1:S434–S435.
Article26. Chiu YC, Chen CC, Ko WC, Liao SC, Yeh HZ, Chang CH. Real‐ world efficacy and safety of vedolizumab among patients with inflammatory bowel disease: a single tertiary medical center experience in Central Taiwan. Adv Dig Med. [published online ahead of print February 19, 2020]. https://doi.org/10.1002/aid2.13188.
Article27. Kim J, Ham NS, Oh EH, et al. Real life effectiveness and safety of vedolizumab induction and maintenance therapy for Korean IBD patients in whom anti-TNF treatment failed: a prospective cohort study. J Crohns Colitis. 2019; 13 Suppl 1:S237.28. Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013; 108:1268–1276.
Article29. Hong SN, Kim HJ, Kim KH, Han SJ, Ahn IM, Ahn HS. Risk of incident Mycobacterium tuberculosis infection in patients with inflammatory bowel disease: a nationwide population-based study in South Korea. Aliment Pharmacol Ther. 2017; 45:253–263.
Article30. Mañosa M, Domènech E, Cabré E. Current incidence of active tuberculosis in IBD patients treated with anti-TNF agents: still room for improvement. J Crohns Colitis. 2013; 7:e499–e500.
Article31. Byun JM, Lee CK, Rhee SY, et al. The risk of tuberculosis in Korean patients with inflammatory bowel disease receiving tumor necrosis factor-α blockers. J Korean Med Sci. 2015; 30:173–179.
Article32. Jauregui-Amezaga A, Turon F, Ordás I, et al. Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J Crohns Colitis. 2013; 7:208–212.
Article33. Puri AS, Desai D, Sood A, Sachdeva S. Infliximab-induced tuberculosis in patients with UC: experience from India-a country with high prevalence of tuberculosis. J Gastroenterol Hepatol. 2017; 32:1191–1194.
Article34. Navarra SV, Raso A, Lichauco JJ, Tan PP. Clinical experience with infliximab among Filipino patients with rheumatic diseases. APLAR J Rheumatol. 2006; 9:150–156.
Article35. Carpio D, Jauregui-Amezaga A, de Francisco R, et al. Tuberculosis in anti-tumour necrosis factor-treated inflammatory bowel disease patients after the implementation of preventive measures: compliance with recommendations and safety of retreatment. J Crohns Colitis. 2016; 10:1186–1193.
Article36. World Health Organization. Global tuberculosis report 2017 [Internet]. c2017 [cited 2018 Mar 30]. http://www.who.int/tb/publications/global_report/en/.37. Shan S, Cui F, Jia J. How to control highly endemic hepatitis B in Asia. Liver Int. 2018; 38 Suppl 1:122–125.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and safety of vedolizumab in ulcerative colitis in patients from Asian countries in the GEMINI 1 study
- Population pharmacokinetics of vedolizumab in Asian and non-Asian patients with ulcerative colitis and Crohn’s disease
- Old and New Biologics and Small Molecules in Inflammatory Bowel Disease: Anti Integrins
- The Efficacy of Vedolizumab, an alpha4beta7 Integrin Antibody, in Crohn's Disease and Ulcerative Colitis
- Treatment of inflammatory bowel diseases: focusing on biologic agents and new therapies