Nutr Res Pract.
2021 Feb;15(1):12-25. 10.4162/nrp.2021.15.1.12.
Synergistic anticancer activity of resveratrol in combination with docetaxel in prostate carcinoma cells
- Affiliations
-
- 1Department of Biochemistry, College of Medicine, Soonchunhyang University, Cheonan 31151, Korea
- KMID: 2511669
- DOI: http://doi.org/10.4162/nrp.2021.15.1.12
Abstract
- BACKGROUND/OBJECTIVES
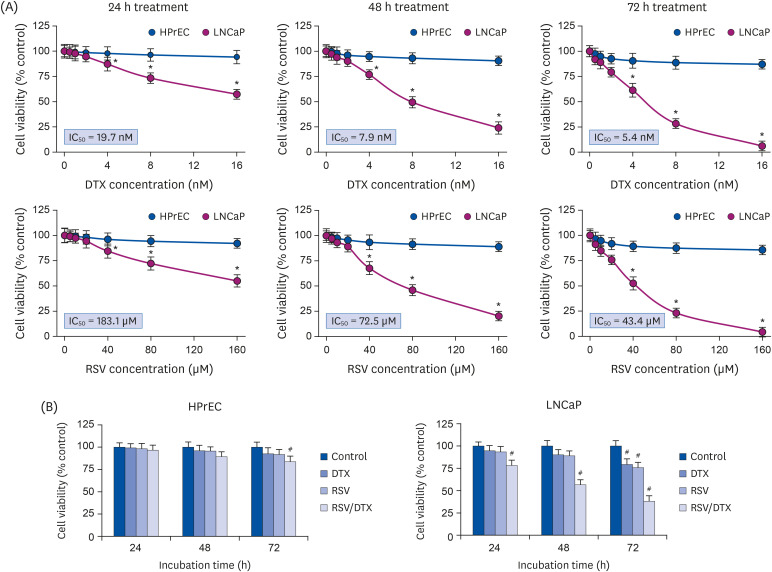
The study was conducted to investigate the efficacy of the combination treatment of phytochemical resveratrol and the anticancer drug docetaxel (DTX) on prostate carcinoma LNCaP cells, including factors related to detailed cell death mechanisms.
MATERIALS/METHODS
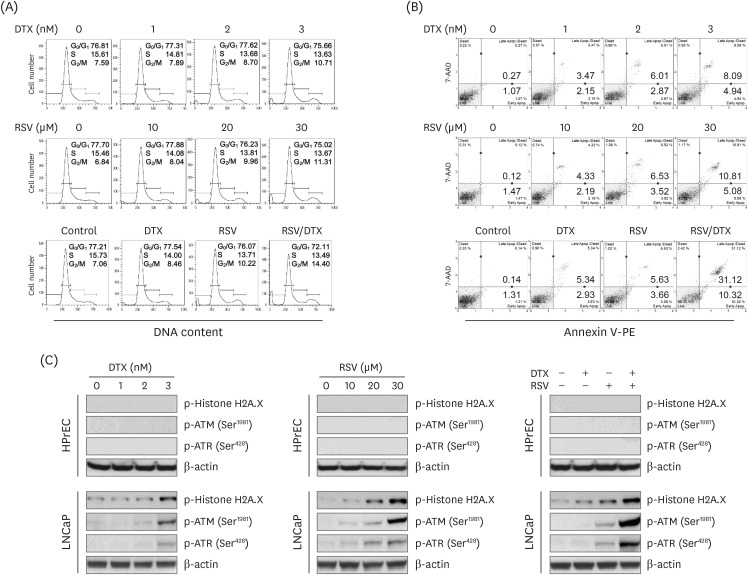
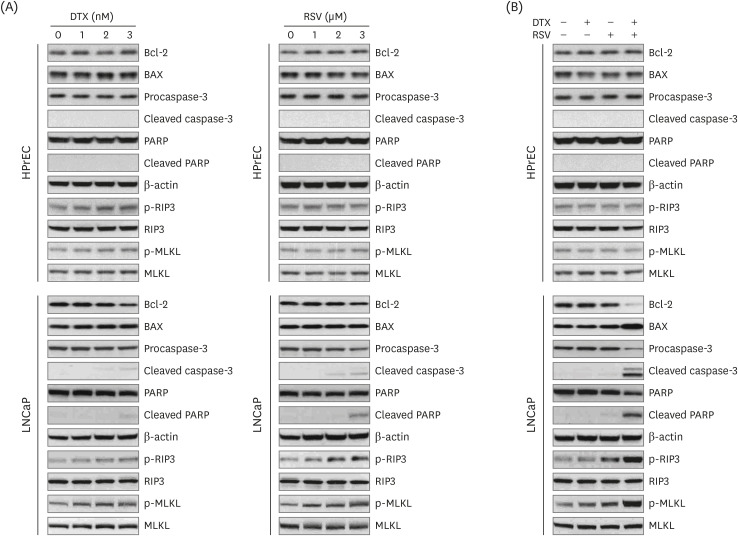
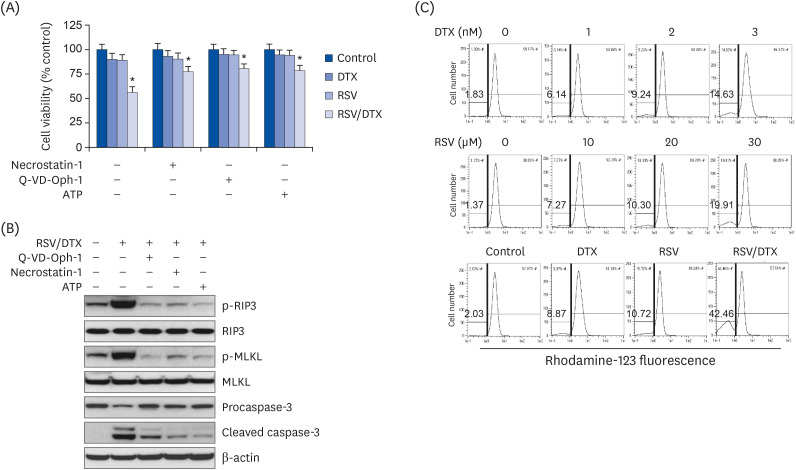
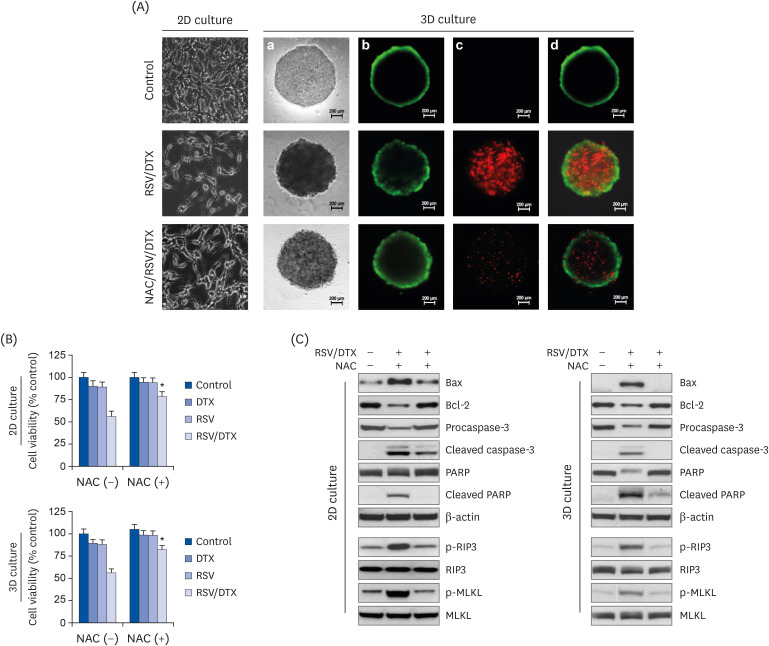
Using 2-dimensional monolayer and 3-dimensional spheroid culture systems, we examined the effects of resveratrol and DTX on cell viability, reactive oxygen species (ROS) levels, mitochondrial membrane potential, apoptosis, and necroptosis by MTT, flow cytometry, and Western blotting.
RESULTS
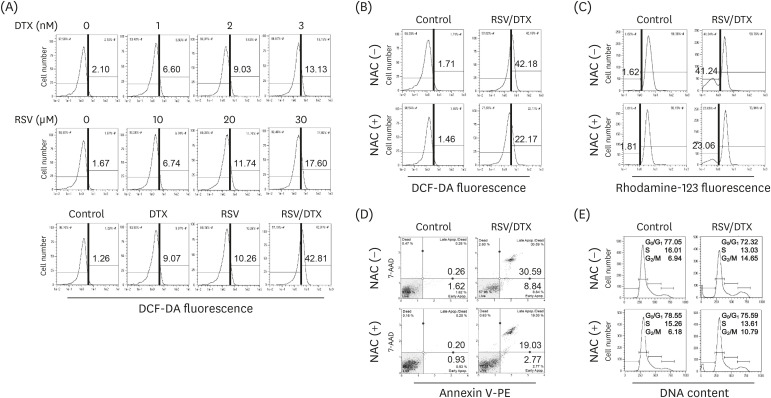
At concentrations not toxic to normal human prostate epithelial cells, resveratrol effectively decreased the viability of LNCaP cells depending on concentration and time. The combination treatment of resveratrol and DTX exhibited synergistic inhibitory effects on cell growth, demonstrated by an increase in the sub-G0/G1 peak, Annexin V-phycoerythrin positive cell fraction, ROS, mitochondrial dysfunction, and DNA damage response as well as concurrent activation of apoptosis and necroptosis. Apoptosis and necroptosis were rescued by pretreatment with ROS scavenger N-acetylcysteine.
CONCLUSIONS
We report resveratrol as an adjuvant drug candidate for improving the outcome of treatment in DTX therapy. Although the underlying mechanisms of necroptosis should be investigated comprehensively, targeting apoptosis and necroptosis simultaneously in the treatment of cancer can be a useful strategy for the development of promising drug candidates.
Keyword
Figure
Reference
-
1. War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC. Mechanisms of plant defense against insect herbivores. Plant Signal Behav. 2012; 7:1306–1320. PMID: 22895106.
Article2. Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, Fokou PV, Martins N, Sharifi-Rad J. Resveratrol: a double-edged sword in health benefits. Biomedicines. 2018; 6:91.
Article3. Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017; 18:2589.
Article4. Takashina M, Inoue S, Tomihara K, Tomita K, Hattori K, Zhao QL, Suzuki T, Noguchi M, Ohashi W, Hattori Y. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. Int J Oncol. 2017; 50:787–797. PMID: 28197625.
Article5. Hu S, Li X, Xu R, Ye L, Kong H, Zeng X, Wang H, Xie W. The synergistic effect of resveratrol in combination with cisplatin on apoptosis via modulating autophagy in A549 cells. Acta Biochim Biophys Sin (Shanghai). 2016; 48:528–535. PMID: 27084520.
Article6. Chang TW, Lin CY, Tzeng YJ, Lur HS. Synergistic combinations of tanshinone IIA and trans-resveratrol toward cisplatin-comparable cytotoxicity in HepG2 human hepatocellular carcinoma cells. Anticancer Res. 2014; 34:5473–5480. PMID: 25275043.7. Kai L, Levenson AS. Combination of resveratrol and antiandrogen flutamide has synergistic effect on androgen receptor inhibition in prostate cancer cells. Anticancer Res. 2011; 31:3323–3330. PMID: 21965742.8. Singh SK, Banerjee S, Acosta EP, Lillard JW, Singh R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget. 2017; 8:17216–17228. PMID: 28212547.9. Sharma A, Boise LH, Shanmugam M. Cancer metabolism and the evasion of apoptotic cell death. Cancers (Basel). 2019; 11:1144.
Article10. Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006; 20:1–15. PMID: 16391229.
Article11. Su Z, Yang Z, Xie L, DeWitt JP, Chen Y. Cancer therapy in the necroptosis era. Cell Death Differ. 2016; 23:748–756. PMID: 26915291.
Article12. Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan K, Cheng H, Jin K, Ni Q, Yu X, Liu C. The role of necroptosis in cancer biology and therapy. Mol Cancer. 2019; 18:100. PMID: 31122251.
Article13. Adachi I, Watanabe T, Takashima S, Narabayashi M, Horikoshi N, Aoyama H, Taguchi T. A late phase II study of RP56976 (docetaxel) in patients with advanced or recurrent breast cancer. Br J Cancer. 1996; 73:210–216. PMID: 8546908.
Article14. Hernández-Vargas H, Palacios J, Moreno-Bueno G. Telling cells how to die: docetaxel therapy in cancer cell lines. Cell Cycle. 2007; 6:780–783. PMID: 17377494.
Article15. Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H. Combination therapy in combating cancer. Oncotarget. 2017; 8:38022–38043. PMID: 28410237.
Article16. Tsakalozou E, Eckman AM, Bae Y. Combination effects of docetaxel and doxorubicin in hormone-refractory prostate cancer cells. Biochem Res Int. 2012; 2012:832059. PMID: 22811914.
Article17. Petrylak DP. Docetaxel for the treatment of hormone-refractory prostate cancer. Rev Urol. 2003; 5 Suppl 2:S14–21.18. Savarese DM, Halabi S, Hars V, Akerley WL, Taplin ME, Godley PA, Hussain A, Small EJ, Vogelzang NJ. Phase II study of docetaxel, estramustine, and low-dose hydrocortisone in men with hormone-refractory prostate cancer: a final report of CALGB 9780. J Clin Oncol. 2001; 19:2509–2516. PMID: 11331330.
Article19. Budman DR, Calabro A, Kreis W. Synergistic and antagonistic combinations of drugs in human prostate cancer cell lines in vitro . Anticancer Drugs. 2002; 13:1011–1016. PMID: 12439335.20. Lee YJ, Lee YJ, Im JH, Won SY, Kim YB, Cho MK, Nam HS, Choi YJ, Lee SH. Synergistic anti-cancer effects of resveratrol and chemotherapeutic agent clofarabine against human malignant mesothelioma MSTO-211H cells. Food Chem Toxicol. 2013; 52:61–68. PMID: 23146690.
Article21. Chambers KF, Mosaad EM, Russell PJ, Clements JA, Doran MR 3rd. 3D cultures of prostate cancer cells cultured in a novel high-throughput culture platform are more resistant to chemotherapeutics compared to cells cultured in monolayer. PLoS One. 2014; 9:e111029. PMID: 25380249.
Article22. Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997; 57:1835–1840. PMID: 9157970.23. Bray K, Chen HY, Karp CM, May M, Ganesan S, Karantza-Wadsworth V, DiPaola RS, White E. Bcl-2 modulation to activate apoptosis in prostate cancer. Mol Cancer Res. 2009; 7:1487–1496. PMID: 19737977.
Article24. Zhang XQ, Huang XF, Hu XB, Zhan YH, An QX, Yang SM, Xia AJ, Yi J, Chen R, Mu SJ, Wu DC. Apogossypolone, a novel inhibitor of antiapoptotic Bcl-2 family proteins, induces autophagy of PC-3 and LNCaP prostate cancer cells in vitro . Asian J Androl. 2010; 12:697–708. PMID: 20657602.25. Wang X, Jiang W, Yan Y, Gong T, Han J, Tian Z, Zhou R. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol. 2014; 15:1126–1133. PMID: 25326752.
Article26. Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014; 54:133–146. PMID: 24703947.
Article27. Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, Zhang Y, Ma H, Huang D, Li W, Xia Z, Han J. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J Biol Chem. 2013; 288:16247–16261. PMID: 23612963.
Article28. Cheung-Ong K, Giaever G, Nislow C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol. 2013; 20:648–659. PMID: 23706631.
Article29. Schmitt E, Paquet C, Beauchemin M, Bertrand R. DNA-damage response network at the crossroads of cell-cycle checkpoints, cellular senescence and apoptosis. J Zhejiang Univ Sci B. 2007; 8:377–397. PMID: 17565509.
Article30. Pearl LH, Schierz AC, Ward SE, Al-Lazikani B, Pearl FM. Therapeutic opportunities within the DNA damage response. Nat Rev Cancer. 2015; 15:166–180. PMID: 25709118.
Article31. Borges HL, Linden R, Wang JY. DNA damage-induced cell death: lessons from the central nervous system. Cell Res. 2008; 18:17–26. PMID: 18087290.
Article32. Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992; 267:5317–5323. PMID: 1312087.
Article33. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016; 1863:2977–2992. PMID: 27646922.
Article34. Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014; 15:411–421. PMID: 24854789.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resveratrol from Peanut Sprout Extract Promotes NK Cell Activation and Antitumor Activity
- Resveratrol Promotes Self-digestion to Put Cancer to Sleep
- Resveratrol Inhibits IL-6-Induced Transcriptional Activity of AR and STAT3 in Human Prostate Cancer LNCaP-FGC Cells
- Resveratrol inhibits cell growth via targeting the Bmi-1 pathway in YD-10B human oral squamous cell carcinoma cells
- Apoptotic effect of IP6 was not enhanced by co-treatment with myo-inositol in prostate carcinoma PC3 cells