J Pathol Transl Med.
2021 Jan;55(1):1-15. 10.4132/jptm.2020.11.20.
Standardized pathology report for breast cancer
- Affiliations
-
- 1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 3Department of Pathology, Yeungnam University College of Medicine, Daegu, Korea
- 4Department of Pathology, Pusan National University Yangsan Hospital, Pusan National University College of Medicine, Yangsan, Korea
- 5Department of Pathology, Eulji University College of Medicine, Seoul, Korea
- 6Department of Pathology, Inje University Haeundae Paik Hospital, Busan, Korea
- 7Department of Pathology, National Cancer Center, Goyang, Korea
- 8Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 9Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 10Department of Pathology, Chonnam National University Medical School, Gwangju, Korea
- 11Department of Pathology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, Korea
- 12Department of Pathology, Busan Paik Hospital, Inje University, Busan, Korea
- KMID: 2511508
- DOI: http://doi.org/10.4132/jptm.2020.11.20
Abstract
- Given the recent advances in management and understanding of breast cancer, a standardized pathology report reflecting these changes is critical. To meet this need, the Breast Pathology Study Group of the Korean Society of Pathologists has developed a standardized pathology reporting format for breast cancer, consisting of ‘standard data elements,’ ‘conditional data elements,’ and a biomarker report form. The ‘standard data elements’ consist of the basic pathologic features used for prognostication, while other factors related to prognosis or diagnosis are described in the ‘conditional data elements.’ In addition to standard data elements, all recommended issues are also presented. We expect that this standardized pathology report for breast cancer will improve diagnostic concordance and communication between pathologists and clinicians, as well as between pathologists inter-institutionally.
Keyword
Figure
Reference
-
References
1. Kang SY, Kim YS, Kim Z, et al. Breast cancer statistics in Korea in 2017: data from a breast cancer registry. J Breast Cancer. 2020; 23:115–28.
Article2. Li X, Oprea-Ilies GM, Krishnamurti U. New developments in breast cancer and their impact on daily practice in pathology. Arch Pathol Lab Med. 2017; 141:490–8.
Article3. Fitzgibbons PL, Connolly JL, Bose S, et al. Protocol for the examination of resection specimens from patients with invasive carcinoma of the breast [Internet]. Northfield: College of American Pathologists;c2020 [cited 2020 Sep 10]. Available from: https://documents.cap.org/protocols/cp-breast-invasive-resection-20-4400.pdf.4. Hortobagyi GN, Connolly JL, D'Orsi CJ, et al. Breast. In : Amin MB, Edge S, Greene F, editors. AJCC cancer staging manual. 8th ed. New York: Springer;2017. p. 589–636.5. WHO Classification of Tumours Editorial Board. WHO classification of tumours: breast tumours. 5th ed. Lyon: International Agency for Research on Cancer;2019.6. Lester SC, Bose S, Chen YY, et al. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch Pathol Lab Med. 2009; 133:1515–38.
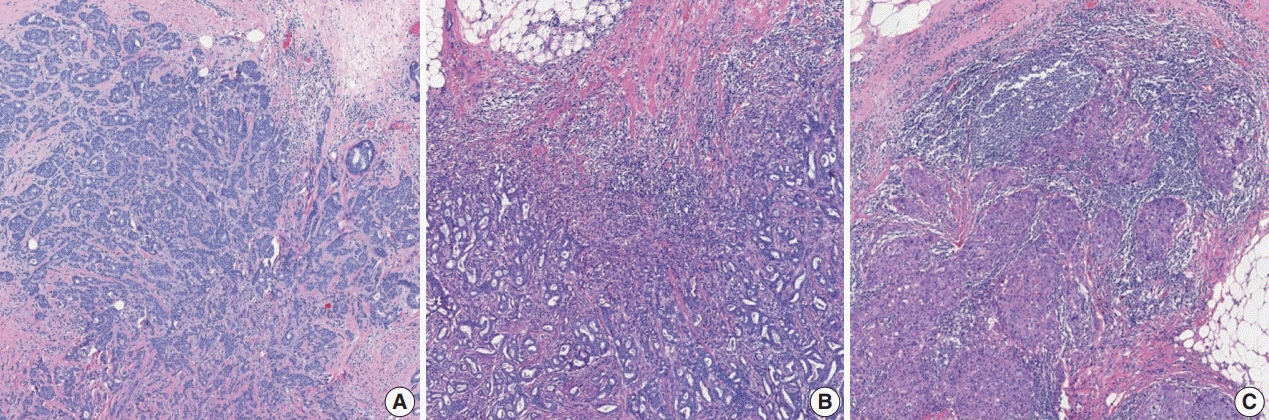
Article7. Salgado R, Aftimos P, Sotiriou C, Desmedt C. Evolving paradigms in multifocal breast cancer. Semin Cancer Biol. 2015; 31:111–8.
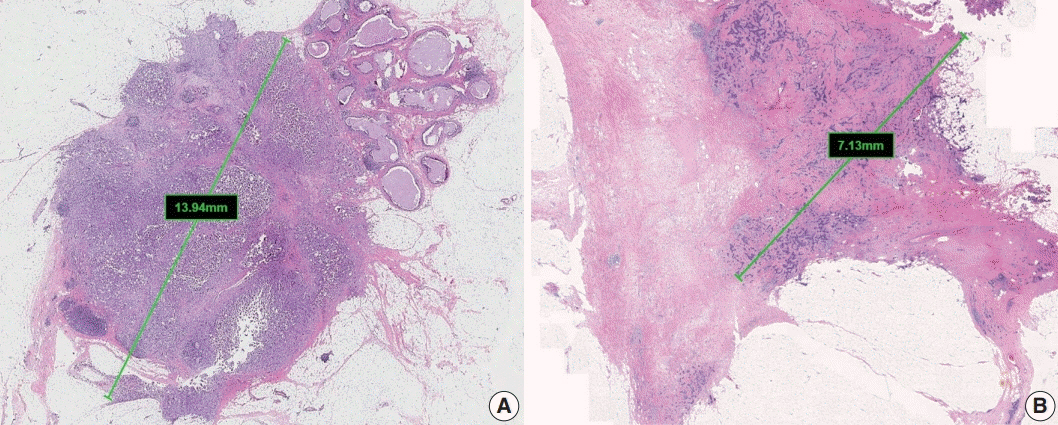
Article8. Andea AA, Wallis T, Newman LA, Bouwman D, Dey J, Visscher DW. Pathologic analysis of tumor size and lymph node status in multifocal/multicentric breast carcinoma. Cancer. 2002; 94:1383–90.
Article9. Weissenbacher TM, Zschage M, Janni W, et al. Multicentric and multifocal versus unifocal breast cancer: is the tumor-node-metastasis classification justified? Breast Cancer Res Treat. 2010; 122:27–34.
Article10. Coombs NJ, Boyages J. Multifocal and multicentric breast cancer: does each focus matter? J Clin Oncol. 2005; 23:7497–502.
Article11. Choi Y, Kim EJ, Seol H, et al. The hormone receptor, human epidermal growth factor receptor 2, and molecular subtype status of individual tumor foci in multifocal/multicentric invasive ductal carcinoma of breast. Hum Pathol. 2012; 43:48–55.
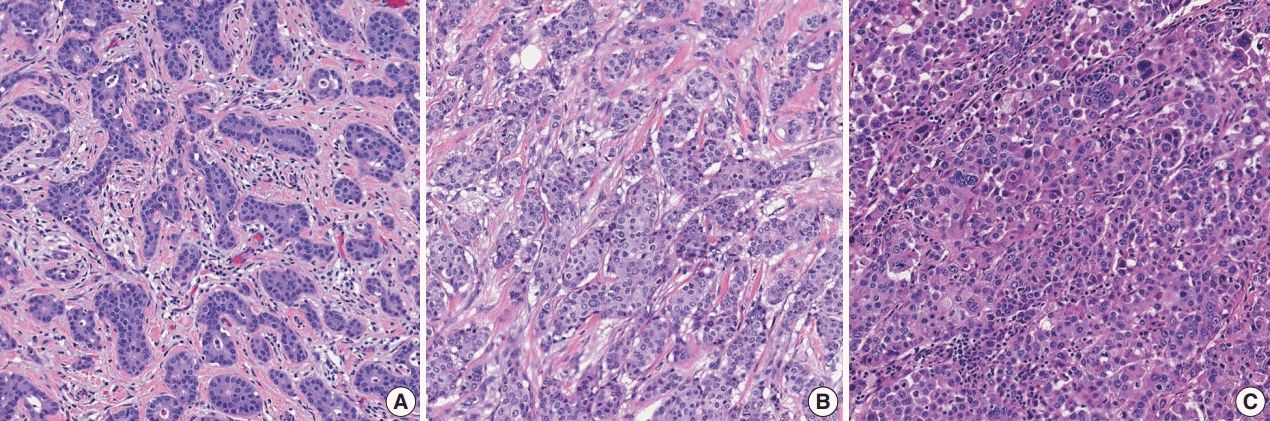
Article12. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–10.
Article13. Rakha EA, El-Sayed ME, Lee AH, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008; 26:3153–8.
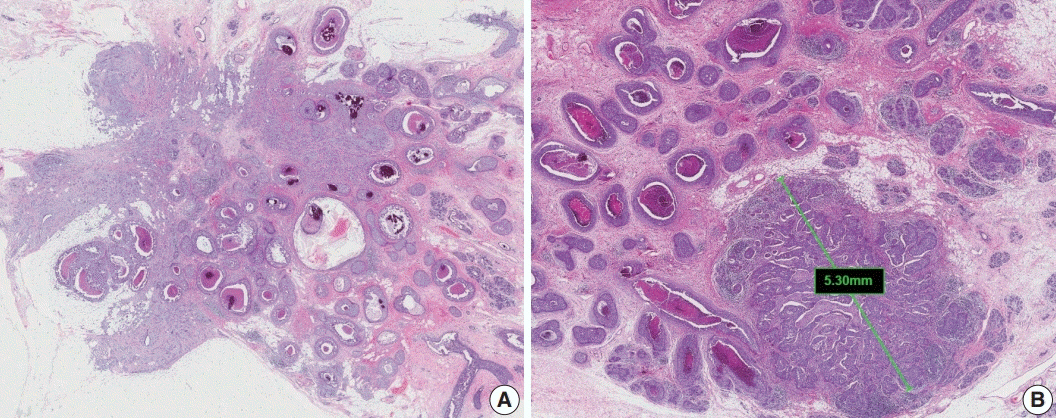
Article14. Jaffer S, Bleiweiss IJ. Histologic classification of ductal carcinoma in situ. Microsc Res Tech. 2002; 59:92–101.
Article15. Grin A, Horne G, Ennis M, O'Malley FP. Measuring extent of ductal carcinoma in situ in breast excision specimens: a comparison of 4 methods. Arch Pathol Lab Med. 2009; 133:31–7.
Article16. Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Pract Radiat Oncol. 2016; 6:287–95.
Article17. Choi AH, Blount S, Perez MN, et al. Size of extranodal extension on sentinel lymph node dissection in the American College of Surgeons Oncology Group Z0011 trial era. JAMA Surg. 2015; 150:1141–8.
Article18. Mohammed RA, Martin SG, Mahmmod AM, et al. Objective assessment of lymphatic and blood vascular invasion in lymph nodenegative breast carcinoma: findings from a large case series with long-term follow-up. J Pathol. 2011; 223:358–65.
Article19. Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol. 2015; 28:1185–201.
Article20. Baker GM, King TA, Schnitt SJ. Evaluation of breast and axillary lymph node specimens in breast cancer patients treated with neoadjuvant systemic therapy. Adv Anat Pathol. 2019; 26:221–34.
Article21. Hoda SA, Brogi E, Koerner FC, Rosen PP. Rosen's breast pathology. Philadelphia: Wolters Kluwer;2014.22. Duraker N, Caynak ZC, Turkoz K. Perineural invasion has no prognostic value in patients with invasive breast carcinoma. Breast. 2006; 15:629–34.
Article23. McCready DR, Chapman JA, Hanna WM, et al. Factors affecting distant disease-free survival for primary invasive breast cancer: use of a log-normal survival model. Ann Surg Oncol. 2000; 7:416–26.
Article24. McCready DR, Chapman JA, Hanna WM, et al. Factors associated with local breast cancer recurrence after lumpectomy alone: postmenopausal patients. Ann Surg Oncol. 2000; 7:562–7.
Article25. Owings DV, Hann L, Schnitt SJ. How thoroughly should needle localization breast biopsies be sampled for microscopic examination? A prospective mammographic/pathologic correlative study. Am J Surg Pathol. 1990; 14:578–83.26. Silverstein MJ, Lagios MD, Recht A, et al. Image-detected breast cancer: state of the art diagnosis and treatment. J Am Coll Surg. 2005; 201:586–97.
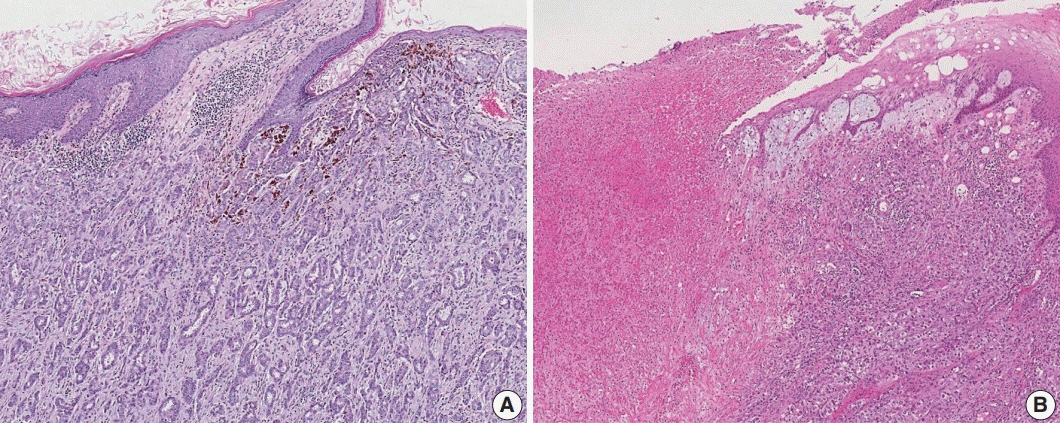
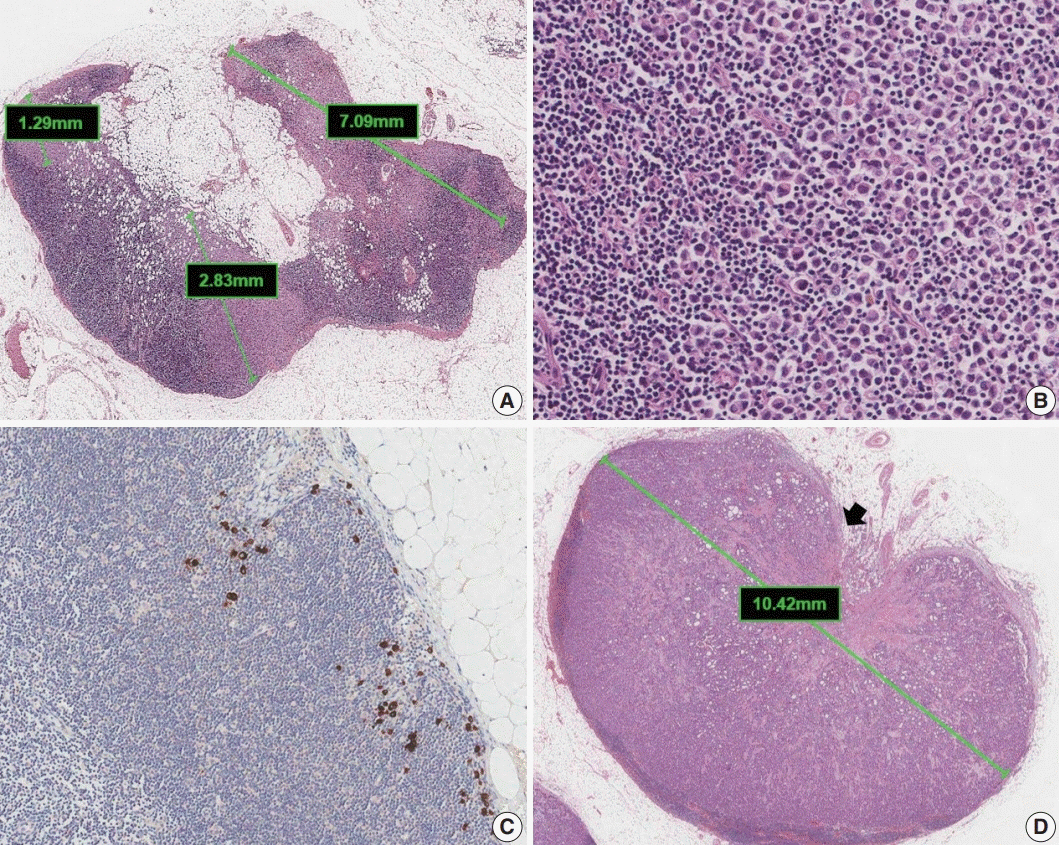
Article27. Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992; 28a:859–64.
Article28. Wein L, Savas P, Luen SJ, Virassamy B, Salgado R, Loi S. Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol. 2017; 7:156.
Article29. Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017; 24:311–35.
Article30. Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumorinfiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018; 52:16–25.
Article31. Denkert C, Wienert S, Poterie A, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016; 29:1155–64.
Article32. Kos Z, Roblin E, Kim RS, et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer. 2020; 6:17.33. Chevallier B, Roche H, Olivier JP, Chollet P, Hurteloup P. Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. Am J Clin Oncol. 1993; 16:223–8.34. Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg. 1995; 180:297–306.35. Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002; 95:681–95.36. Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003; 12:320–7.
Article37. Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007; 25:4414–22.
Article38. Mittendorf EA, Jeruss JS, Tucker SL, et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011; 29:1956–62.
Article39. Chollet P, Abrial C, Durando X, et al. A new prognostic classification after primary chemotherapy for breast cancer: residual disease in breast and nodes (RDBN). Cancer J. 2008; 14:128–32.
Article40. Mrkonjic M, Berman HK, Done SJ, Youngson B, Mulligan AM. Breast specimen handling and reporting in the post-neoadjuvant setting: challenges and advances. J Clin Pathol. 2019; 72:120–32.
Article41. Bossuyt V. Processing and reporting of breast specimens in the neoadjuvant setting. Surg Pathol Clin. 2018; 11:213–30.
Article42. Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005; 23:9304–11.
Article43. von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–804.
Article44. Pinder SE, Provenzano E, Earl H, Ellis IO. Laboratory handling and histology reporting of breast specimens from patients who have received neoadjuvant chemotherapy. Histopathology. 2007; 50:409–17.
Article45. Smith IC, Heys SD, Hutcheon AW, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002; 20:1456–66.
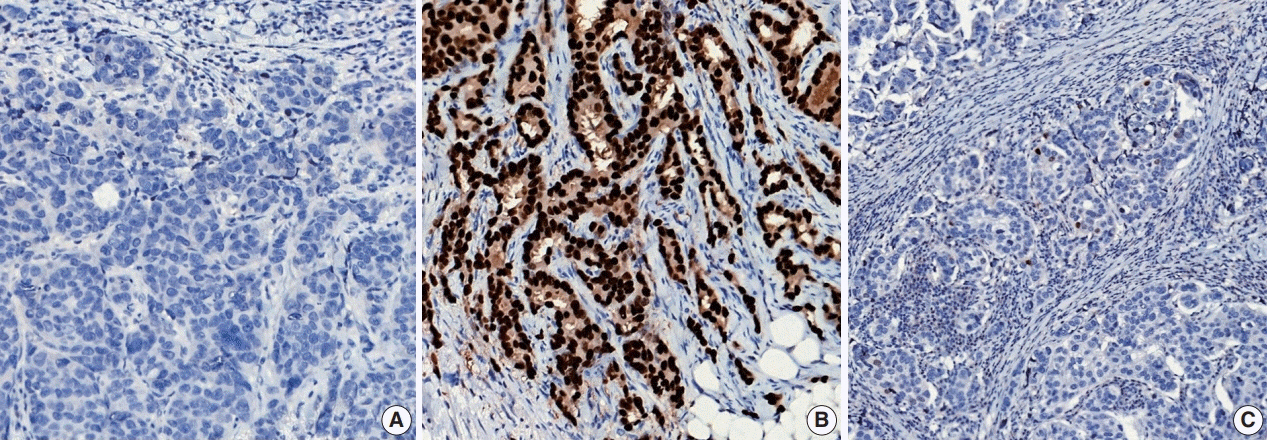
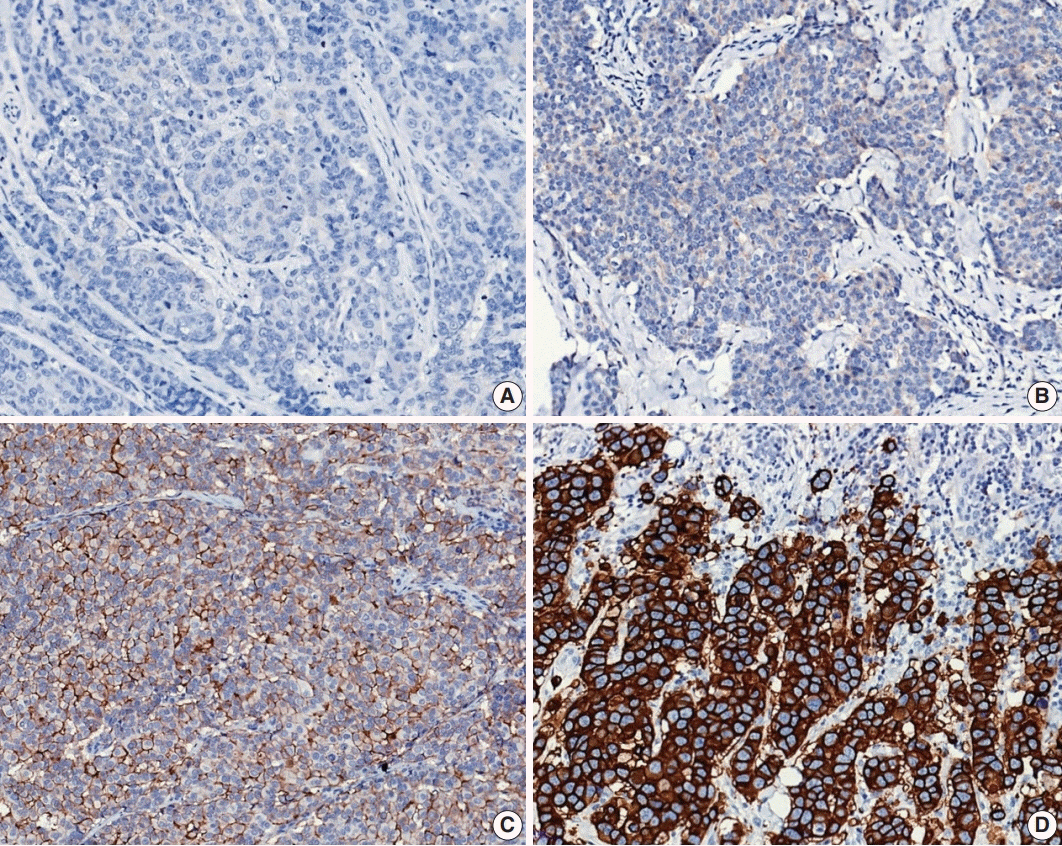
Article46. Allison KH, Hammond ME, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020; 38:1346–66.
Article47. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018; 36:2105–22.48. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999; 17:1474–81.
Article49. Ahn S, Woo JW, Lee K, Park SY. HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. J Pathol Transl Med. 2020; 54:34–44.
Article50. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.51. Luporsi E, Andre F, Spyratos F, et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012; 132:895–915.
Article52. Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010; 11:174–83.
Article53. Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008; 26:5569–75.
Article54. Penault-Llorca F, André F, Sagan C, et al. Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009; 27:2809–15.
Article55. Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011; 103:1656–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Standardized Pathology Report for Breast Cancer
- The Highligts of 28th Annual Meeting of San Antonio Breast Cancer Symposium
- HER-2/neu in Breast Cancer: Is Consensus Reached in Standard Testing?
- Surgery of Breast Cancer during the Last 5 Years: More Sophisticated and Specialized?
- Carcinoma of the axillary breast