Clin Endosc.
2020 Nov;53(6):652-658. 10.5946/ce.2019.184.
Present Status of Endoscopic Submucosal Dissection for Non-Ampullary Duodenal Epithelial Tumors
- Affiliations
-
- 1Division of Endoscopy, Shizuoka Cancer Center, Shizuoka, Japan
- KMID: 2511224
- DOI: http://doi.org/10.5946/ce.2019.184
Abstract
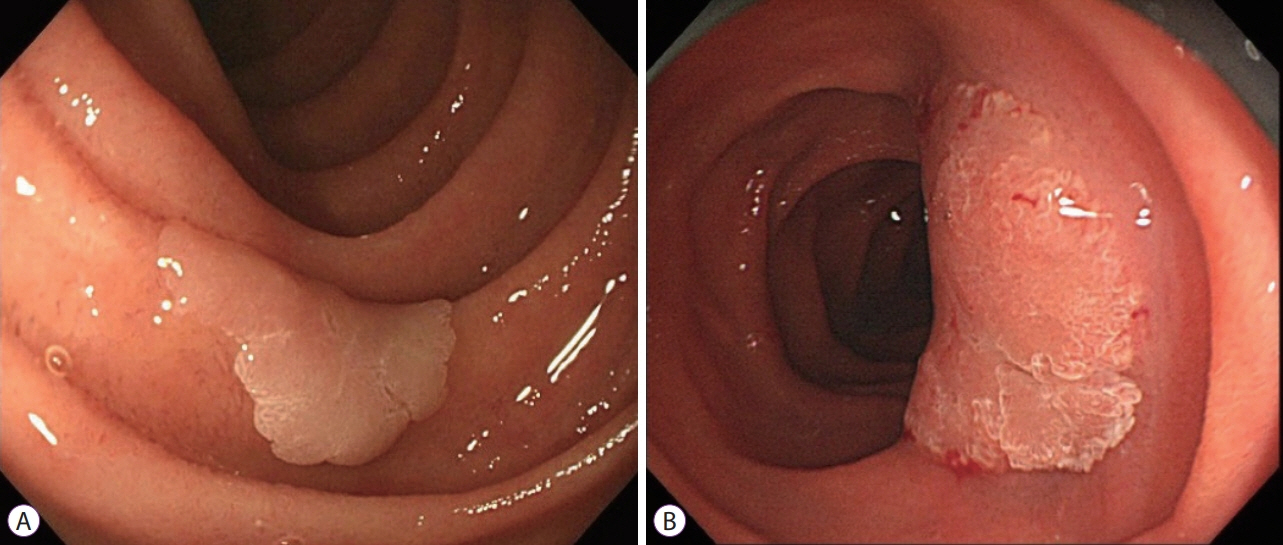
- Prediction of histology by endoscopic examination is important in the clinical management of non-ampullary duodenal epithelial tumors (NADETs), including adenoma and adenocarcinoma. The use of a simple scoring system based on the findings of white-light endoscopy or magnified endoscopy with narrow-band imaging is useful to differentiate between Vienna category 3 (C3) and C4/5 lesions. Less invasive endoscopic resection procedures, such as cold snare polypectomy, are quick to perform and convenient for small (<10 mm) C3 lesions. Neoplasms with higher grade histology, such as C4/5 lesions, should be treated by endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), or surgery. Although EMR often requires piecemeal resection, the complication rate is acceptable. Excellent complete resection rates could be achieved by ESD; however, it remains a challenging method considering the high risk of complications. Shielding or closure of the ulcer after ESD is effective at decreasing the risk of delayed bleeding and perforation. Laparoscopic endoscopic cooperative surgery is an ideal treatment with a high rate of en bloc resection and a low rate of complications, although it is limited to high-volume centers. Patients with NADETs could benefit from a multidisciplinary approach to stratify the optimal treatment based on endoscopic diagnoses.
Keyword
Figure
Cited by 1 articles
-
Endoscopic Treatment for Superficial Nonampullary Duodenal Tumors
Hyo-Joon Yang
Korean J Gastroenterol. 2021;77(4):164-170. doi: 10.4166/kjg.2021.039.
Reference
-
1. Yoshimura N, Goda K, Tajiri H, Ikegami M, Nakayoshi T, Kaise M. Endoscopic features of nonampullary duodenal tumors with narrow-band imaging. Hepatogastroenterology. 2010; 57:462–467.2. Goda K, Kikuchi D, Yamamoto Y, et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: multicenter case series. Dig Endosc. 2014; 26 Suppl 2:23–29.
Article3. Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002; 51:130–131.
Article4. Kakushima N, Yoshida M, Iwai T, et al. A simple endoscopic scoring system to differentiate between duodenal adenoma and carcinoma. Endosc Int Open. 2017; 5:E763–E768.
Article5. Kikuchi D, Hoteya S, Iizuka T, Kimura R, Kaise M. Diagnostic algorithm of magnifying endoscopy with narrow band imaging for superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014; 26 Suppl 2:16–22.
Article6. Kakushima N, Yoshida M, Yamaguchi Y, et al. Magnified endoscopy with narrow-band imaging for the differential diagnosis of superficial non-ampullary duodenal epithelial tumors. Scand J Gastroenterol. 2019; 54:128–134.
Article7. Kakushima N, Kanemoto H, Tanaka M, Takizawa K, Ono H. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014; 20:12501–12508.
Article8. Maruoka D, Matsumura T, Kasamatsu S, et al. Cold polypectomy for duodenal adenomas: a prospective clinical trial. Endoscopy. 2017; 49:776–783.
Article9. Hamada K, Takeuchi Y, Ishikawa H, et al. Feasibility of cold snare polypectomy for multiple duodenal adenomas in patients with familial adenomatous polyposis: a pilot study. Dig Dis Sci. 2016; 61:2755–2759.
Article10. Ichihara S, Uraoka T, Oka S. Challenges associated with the pathological diagnosis of colorectal tumors less than 10 mm in size. Dig Endosc. 2018; 30 Suppl 1:41–44.11. Lee CK, Shim JJ, Jang JY. Cold snare polypectomy vs. cold forceps polypectomy using double-biopsy technique for removal of diminutive colorectal polyps: a prospective randomized study. Am J Gastroenterol. 2013; 108:1593–1600.
Article12. Binmoeller KF, Shah JN, Bhat YM, Kane SD. “Underwater” EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video). Gastrointest Endosc. 2013; 78:496–502.
Article13. Binmoeller KF. Underwater EMR without submucosal injection: is less more? Gastrointest Endosc. 2019; 89:1117–1119.
Article14. Yamasaki Y, Uedo N, Takeuchi Y, et al. Underwater endoscopic mucosal resection for superficial nonampullary duodenal adenomas. Endoscopy. 2018; 50:154–158.
Article15. Kato M, Tsujii Y, Takehara T. Underwater endoscopic mucosal resection of a duodenal adenoma with biopsy scars. Dig Endosc. 2018; 30:405–406.
Article16. Inoue T, Uedo N, Yamashina T, et al. Delayed perforation: a hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc. 2014; 26:220–227.
Article17. Hoteya S, Kaise M, Iizuka T, et al. Delayed bleeding after endoscopic submucosal dissection for non-ampullary superficial duodenal neoplasias might be prevented by prophylactic endoscopic closure: analysis of risk factors. Dig Endosc. 2015; 27:323–330.
Article18. Doyama H, Tominaga K, Yoshida N, Takemura K, Yamada S. Endoscopic tissue shielding with polyglycolic acid sheets, fibrin glue and clips to prevent delayed perforation after duodenal endoscopic resection. Dig Endosc. 2014; 26 Suppl 2:41–45.
Article19. Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014; 26 Suppl 2:46–49.
Article20. Yahagi N, Nishizawa T, Akimoto T, Ochiai Y, Goto O. New endoscopic suturing method: string clip suturing method. Gastrointest Endosc. 2016; 84:1064–1065.
Article21. Mori H, Shintaro F, Kobara H, et al. Successful closing of duodenal ulcer after endoscopic submucosal dissection with over-the-scope clip to prevent delayed perforation. Dig Endosc. 2013; 25:459–461.
Article22. Toma H, Haraguchi K, Fujii K, Kobarai T, Hirota I, Eguchi T. Laparoscopic and endoscopic cooperative surgery for non-ampullary duodenal epithelial neoplasms. Mini-invasive Surgery. 2018; 2:21.
Article23. Ono H, Kaise M, Nonaka S, et al. [Clinical issues of duodenal endoscopic treatment]. Stomach and Intestine. 2016; 51:1585–1592.24. Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015; 27:417–434.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Resection for Superficial Non-Ampullary Duodenal Epithelial Tumors
- Non-Ampullary Duodenal Tumors
- Usefulness of the S-O clip for duodenal endoscopic submucosal dissection: a propensity score-matched study
- Current Treatment Strategy for Superficial Nonampullary Duodenal Epithelial Tumors
- Underwater Endoscopic Mucosal Resections of Non-ampullary Small Duodenal Tumors