J Korean Med Sci.
2021 Jan;36(3):e31. 10.3346/jkms.2021.36.e31.
Informed Consent for Scholarly Articles during the COVID-19 Pandemic
- Affiliations
-
- 1Fundación Centro, Eating Disorder Institute, Córdoba, Argentina
- 2Department of Internal Medicine No. 2, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
- 3Department Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
- KMID: 2510743
- DOI: http://doi.org/10.3346/jkms.2021.36.e31
Abstract
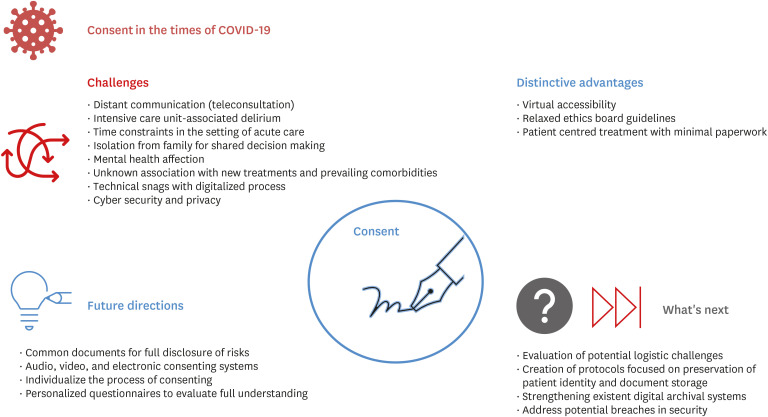
- The coronavirus disease 2019 pandemic has caused a breakdown in the healthcare system worldwide. The need to rapidly update guidelines in order to control the transmission in the population and for evidenced-based healthcare care has led to the need for timely, voluminous and valid research. Amid the quest for a vaccine and better therapies, researchers clamouring for information has led to a wide variety of ethical issues due to the unique situation. This paper aims to examine the positive and negative aspects of recent changes in the process of obtaining informed consent. The article outlines the various aspects, from history, previously described exemptions to consenting as well as those implemented during the pandemic and the current impact of virtual methods. Further, the authors make recommendations based on the outcome of suggested adjustments described in the literature. This article looks into increasing the awareness of physicians and researchers about ethical issues that need to be addressed to provide optimal care for patients while assuring their integrity and confidentiality.
Keyword
Figure
Cited by 1 articles
-
Impact of COVID-19 Pandemic on Biomedical Publications and Their Citation Frequency
Sooyoung Park, Hyun Jeong Lim, Jaero Park, Yeon Hyeon Choe
J Korean Med Sci. 2022;37(40):e296. doi: 10.3346/jkms.2022.37.e296.
Reference
-
1. Whitelaw S, Mamas MA, Topol E, Van Spall HG. Applications of digital technology in COVID-19 pandemic planning and response. Lancet Digit Health. 2020; 2(8):e435–40. PMID: 32835201.
Article2. Monaghesh E, Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020; 20(1):1193. PMID: 32738884.
Article3. Gupta L, Gasparyan AY, Zimba O, Misra DP. Scholarly publishing and journal targeting in the time of the coronavirus disease 2019 (COVID-19) pandemic: a cross-sectional survey of rheumatologists and other specialists. Rheumatol Int. 2020; 40(12):2023–2030. PMID: 33048199.
Article4. Bryan AF, Milner R, Roggin KK, Angelos P, Matthews JB. Unknown unknowns: surgical consent during the COVID-19 pandemic. Ann Surg. 2020; 272(2):e161–2. PMID: 32675526.
Article5. Cocanour CS. Informed consent-it's more than a signature on a piece of paper. Am J Surg. 2017; 214(6):993–997. PMID: 28974311.
Article6. Turnham HL, Dunn M, Hill E, Thornburn GT, Wilkinson D. Consent in the time of COVID-19. J Med Ethics. 2020; 46(9):565–568. PMID: 32522812.
Article7. Tori K, Kalligeros M, Shehadeh F, Khader R, Nanda A, van Aalst R, et al. The process of obtaining informed consent to research in long term care facilities (LTCFs): an Observational Clinical Study. Medicine (Baltimore). 2020; 99(21):e20225. PMID: 32481294.8. Silbert BS, Scott DA. Informed Consent in Patients With Frailty Syndrome. Anesth Analg. 2020; 130(6):1474–1481. PMID: 32384337.
Article9. Zahrai A, Bhanot K, Mei XY, Crawford E, Tan Z, Yee A, et al. Surgeon clinical practice variation and patient preferences during the informed consent discussion: a mixed-methods analysis in lumbar spine surgery. Can J Surg. 2020; 63(3):E284–E291. PMID: 32437095.
Article10. Gabay G, Bokek-Cohen Y. Infringement of the right to surgical informed consent: negligent disclosure and its impact on patient trust in surgeons at public general hospitals - the voice of the patient. BMC Med Ethics. 2019; 20(1):77. PMID: 31660956.
Article11. Corda DM, Dexter F, Pasternak JJ, Trentman TL, Nottmeier EW, Brull SJ. Patients' perspective on full disclosure and informed consent regarding postoperative visual loss associated with spinal surgery in the prone position. Mayo Clin Proc. 2011; 86(9):865–868. PMID: 21878598.
Article12. Misra DP, Agarwal V. Real-world evidence in rheumatic diseases: relevance and lessons learnt. Rheumatol Int. 2019; 39(3):403–416. PMID: 30725156.
Article13. McGuire AL, Aulisio MP, Davis FD, Erwin C, Harter TD, Jagsi R, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the association of bioethics program directors (ABPD) task force. Am J Bioeth. 2020; 20(7):15–27. PMID: 32511078.
Article14. Paul C, Brookes B. The rationalization of unethical research: revisionist accounts of the tuskegee syphilis study and the New Zealand “Unfortunate Experiment”. Am J Public Health. 2015; 105(10):e12–9.
Article15. Misra DP, Agarwal V. To Act…….or to wait for the evidence: Ethics in the time of covid-19! Indian J Rheumatol. 2020; 15(1):3–4.
Article16. Moodley K, Allwood BW, Rossouw TM. Consent for critical care research after death from COVID-19: arguments for a waiver. S Afr Med J. 2020; 110(7):629–634. PMID: 32880337.17. Panda PK, Stockler MR, Gulia A. Clinical research during coronavirus disease pandemic: challenges and way forward. Indian J Med Sci. 2020; 72(2):101–106.
Article18. AlNaamani K, AlSinani S, Barkun AN. Medical research during the COVID-19 pandemic. World J Clin Cases. 2020; 8(15):3156–3163. PMID: 32874970.
Article19. Tuttle KR. Impact of the COVID-19 pandemic on clinical research. Nat Rev Nephrol. 2020; 16(10):562–564. PMID: 32760016.
Article20. Kramer JB, Brown DE, Kopar PK. Ethics in the time of coronavirus: recommendations in the COVID-19 pandemic. J Am Coll Surg. 2020; 230(6):1114–1118. PMID: 32278728.
Article21. Chenneville T, Schwartz-Mette R. Ethical considerations for psychologists in the time of COVID-19. Am Psychol. 2020; 75(5):644–654. PMID: 32437180.
Article22. Kichloo A, Albosta M, Dettloff K, Wani F, El-Amir Z, Singh J, et al. Telemedicine, the current COVID-19 pandemic and the future: a narrative review and perspectives moving forward in the USA. Fam Med Community Health. 2020; 8(3):e000530. PMID: 32816942.
Article23. Gupta L, Misra DP, Agarwal V, Balan S, Agarwal V. Response to: ‘Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort’ by Costa et al. Ann Rhem Dis. Forthcoming 2020. DOI: 10.1136/annrheumdis-2020-217953.
Article24. Haussen DC, Doppelheuer S, Schindler K, Grossberg JA, Bouslama M, Schultz M, et al. Utilization of a smartphone platform for electronic informed consent in acute stroke trials. Stroke. 2017; 48(11):3156–3160. PMID: 28986425.
Article25. U.S. Food and Drug Administration. Part 11, Electronic records; electronic signatures - scope and application. Updated September 2003. Accessed November 21, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/part-11-electronic-records-electronic-signatures-scope-and-application.26. Misra DP, Agarwal V. Blaming the peer reviewer: don't shoot the messenger!! Indian J Rheumatol. 2020; 15(3):162–164.27. Bobb MR, Van Heukelom PG, Faine BA, Ahmed A, Messerly JT, Bell G, et al. Telemedicine provides noninferior research informed consent for remote study enrollment: a randomized controlled trial. Acad Emerg Med. 2016; 23(7):759–765. PMID: 26990899.
Article28. Gupta L, Chinoy H. Monitoring disease activity and damage in adult and juvenile idiopathic inflammatory myopathy. Curr Opin Rheumatol. 2020; 32(6):553–561. PMID: 32890032.
Article29. Davalbhakta S, Advani S, Kumar S, Agarwal V, Bhoyar S, Fedirko E, et al. A systematic review of smartphone applications available for corona virus disease 2019 (COVID19) and the assessment of their quality using the mobile application rating scale (MARS). J Med Syst. 2020; 44(9):164. PMID: 32779002.
Article30. Sneha P. Do India's COVID-19 patients have a right to privacy? Updated July 2020. Accessed November 8, 2020. https://science.thewire.in/health/do-indias-covid-19-patients-have-a-right-to-privacy/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigenda: Omission of the Description of Informed Consent
- Legal Issues Concerning Informed Consent
- Corrigenda: Omission of the Description of Informed Consent on the Identifiable Photos and the Description on Ethical Treatment of Experimental Animals
- The Management of Thyroid Disease in COVID-19 Pandemic
- A Scoping Review of the Effect of the COVID-19 Pandemic on Patients Under Infertility Treatment