J Korean Med Sci.
2021 Jan;36(3):e18. 10.3346/jkms.2021.36.e18.
Safety and Utility of Rush Immunotherapy with Aqueous Allergen Extracts for Treatment of Respiratory Allergies
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- KMID: 2510738
- DOI: http://doi.org/10.3346/jkms.2021.36.e18
Abstract
- Background
Generally, allergen immunotherapy must be administered for three to five years. Meanwhile, rush immunotherapy (RIT) shortens the required duration for the build-up phase, thereby improving the therapy's convenience compared with conventional immunotherapy (CIT). However, RIT is often performed with modified allergens. Therefore, this study aimed to investigate the safety and utility of RIT with aqueous allergens.
Methods
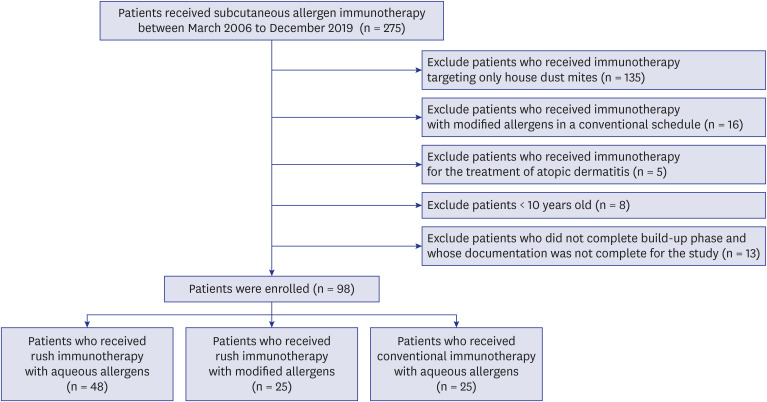
Medical records of 98 patients sensitized with at least one inhalant allergen who had received subcutaneous immunotherapy for allergic rhinitis with or without asthma were retrospectively reviewed. All patients were classified into three groups: depot-RIT (n = 25), receiving RIT with depot allergen; aqueous-RIT (n = 48), receiving RIT with aqueous allergen; and aqueous-CIT (n = 25), receiving CIT with aqueous allergen. Patients who had received immunotherapy targeting only house dust mites were excluded.
Results
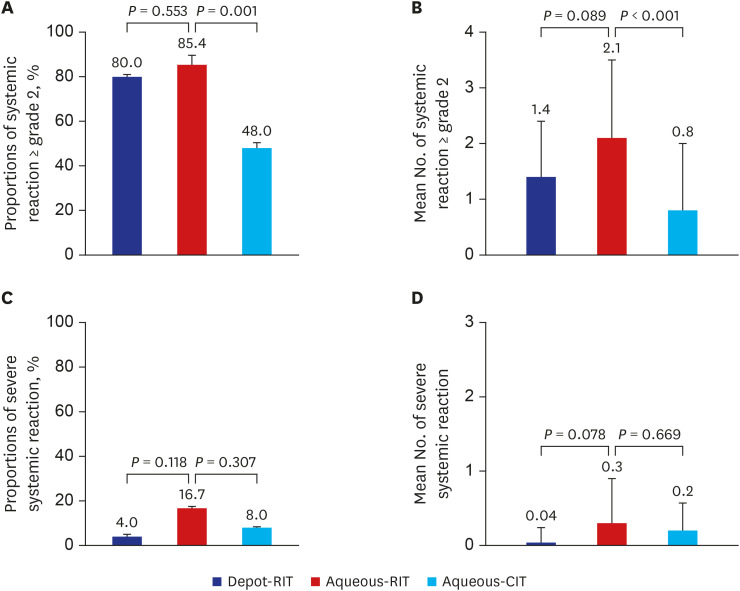
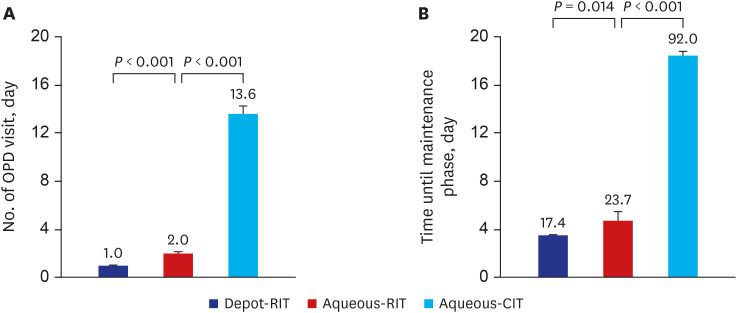
The proportions of patients presenting with a systemic reaction to depot-RIT, aqueous-RIT, or aqueous-CIT were 80.0%, 85.4%, and 48.0%, respectively (P = 0.002). The proportions of patients experiencing severe systemic reaction were 4.0%, 16.7%, and 8.0% in depot-RIT, aqueous-RIT and aqueous-CIT, respectively (P = 0.223). The proportions of depotRIT and aqueous-RIT patients presenting with systemic reaction or severe systemic reaction did not differ significantly (P = 0.553 and P = 0.118, respectively). Significantly fewer depotRIT (1.0 ± 0.2) and aqueous-RIT patients (2.0 ± 1.3) required outpatient clinical visits during the build-up phase, compared to those administered aqueous-CIT (13.6 ± 1.9; P < 0.001).Moreover, the build-up phase decreased to 17.4 ± 1.8 days in depot-RIT and 23.7 ± 10.9 days in aqueous-RIT, compared to 92.0 ± 12.5 days in aqueous-CIT (P < 0.001).
Conclusion
RIT with aqueous allergen reduced the build-up phase duration and frequency of hospital visits, with acceptable safety levels. RIT with aqueous allergen may, therefore, be suitable for broad application to patients with respiratory allergies.
Keyword
Figure
Reference
-
1. Noon L. Prophylactic inoculation against hay fever. Int Arch Allergy Appl Immunol. 1953; 4(4):285–288. PMID: 13096152.
Article2. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015; 136(3):556–568. PMID: 26162571.
Article3. Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007; 62(8):943–948. PMID: 17620073.
Article4. Reha CM, Ebru A. Specific immunotherapy is effective in the prevention of new sensitivities. Allergol Immunopathol (Madr). 2007; 35(2):44–51. PMID: 17428399.
Article5. Rhyou HI, Nam YH. Efficacy of allergen immunotherapy for allergic asthma in real world practice. Allergy Asthma Immunol Res. 2020; 12(1):99–109. PMID: 31743967.
Article6. Shin YS, Jung JW, Park JW, Choi JH, Kwon JW, Lee S, et al. Clinical efficacy of allergen-specific immunotherapy from patient and physician perspectives. Yonsei Med J. 2019; 60(5):446–453. PMID: 31016906.
Article7. Cox LS, Hankin C, Lockey R. Allergy immunotherapy adherence and delivery route: location does not matter. J Allergy Clin Immunol Pract. 2014; 2(2):156–160. PMID: 24607042.
Article8. Lee SP, Choi SJ, Joe E, Lee SM, Lee MW, Shim JW, et al. A pilot study of intralymphatic immunotherapy for house dust mite, cat, and dog allergies. Allergy Asthma Immunol Res. 2017; 9(3):272–277. PMID: 28293934.
Article9. Lee JH, Lee SH, Ban GY, Ye YM, Nahm DH, Park HS, et al. Factors associated with adherence to allergen specific subcutaneous immunotherapy. Yonsei Med J. 2019; 60(6):570–577. PMID: 31124341.
Article10. Hankin CS, Lockey RF. Patient characteristics associated with allergen immunotherapy initiation and adherence. J Allergy Clin Immunol. 2011; 127(1):46–48. 48.e1–48.e3. PMID: 21093021.
Article11. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127(1):Suppl. S1–S55. PMID: 21122901.12. Winslow AW, Turbyville JC, Sublett JW, Sublett JL, Pollard SJ. Comparison of systemic reactions in rush, cluster, and standard-build aeroallergen immunotherapy. Ann Allergy Asthma Immunol. 2016; 117(5):542–545. PMID: 27788885.
Article13. Global Initiative for Asthma. Global strategy for asthma management and prevention. Updated 2020. Accessed May 18, 2020. https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf.14. Sharkey P, Portnoy J. Rush immunotherapy: experience with a one-day schedule. Ann Allergy Asthma Immunol. 1996; 76(2):175–180. PMID: 8595538.
Article15. Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009; 9(4):287–293. PMID: 19247370.
Article16. Pfaar O, Biedermann T, Klimek L, Sager A, Robinson DS. Depigmented-polymerized mixed grass/birch pollen extract immunotherapy is effective in polysensitized patients. Allergy. 2013; 68(10):1306–1313. PMID: 23991896.
Article17. Cox L, Jacobsen L. Comparison of allergen immunotherapy practice patterns in the United States and Europe. Ann Allergy Asthma Immunol. 2009; 103(6):451–459. PMID: 20084837.
Article18. Park KH, Lee SC, Son YW, Jeong KY, Shin YS, Shin JU, et al. Different responses in induction of allergen specific immunoglobulin G4 and IgE-blocking factors for three mite subcutaneous immunotherapy products. Yonsei Med J. 2016; 57(6):1427–1434. PMID: 27593871.
Article19. Passalacqua G. The use of single versus multiple antigens in specific allergen immunotherapy for allergic rhinitis: review of the evidence. Curr Opin Allergy Clin Immunol. 2014; 14(1):20–24. PMID: 24362238.20. Kang MG, Kim MY, Song WJ, Kim S, Jo EJ, Lee SE, et al. Patterns of inhalant allergen sensitization and geographical variation in Korean adults: a multicenter retrospective study. Allergy Asthma Immunol Res. 2017; 9(6):499–508. PMID: 28913989.
Article21. Harvey SM, Laurie S, Hilton K, Khan DA. Safety of rush immunotherapy to multiple aeroallergens in an adult population. Ann Allergy Asthma Immunol. 2004; 92(4):414–419. PMID: 15104192.
Article22. DaVeiga SP, Liu X, Caruso K, Golubski S, Xu M, Lang DM. Systemic reactions associated with subcutaneous allergen immunotherapy: timing and risk assessment. Ann Allergy Asthma Immunol. 2011; 106(6):533–537.e2. PMID: 21624754.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety and efficacy of the ultra-rush immunotherapy with extracts of Dermatophagoides pteronyssinus in children
- Recent advances in food allergen immunotherapy
- Safety of Accelerated Schedules of Subcutaneous Allergen Immunotherapy with House Dust Mite Extract in Patients with Atopic Dermatitis
- Sublingual immunotherapy for allergic rhinitis
- Allergen Immunotherapy: Past, Present, and Future