Kosin Med J.
2020 Dec;35(2):125-132. 10.7180/kmj.2020.35.2.125.
Efficacy of Evolocumab in Patients with Hypercholesterolemia
- Affiliations
-
- 1Department of Cardiology, Dong-A University College of Medicine, Busan, Korea
- KMID: 2510273
- DOI: http://doi.org/10.7180/kmj.2020.35.2.125
Abstract
Objectives
The FOURIER trial reported that inhibition of PCSK9 with evolocumab on a background of statin therapy lowered low-density lipoprotein (LDL) cholesterol levels to a median of 30 mg per deciliter (0.78 mmol per liter) and reduced the risk of cardiovascular events. Here, we report data from a single center focusing on the effect of a PCSK9 inhibitor antibody on hyperlipidemia.
Methods
We enrolled 29 hypercholesterolemia patients who had LDL cholesterol levels ≥ 70 mg per deciliter or nonHDL cholesterol ≥ 100 mg per deciliter and were divided into two groups (placebo n = 14, evolocumab n = 15), and participated in a 72 - 96 week, randomized, double-blind, placebo-controlled trial with statin therapy. Patients were randomly assigned to receive evolocumab (140 mg every 2 weeks or 420 mg monthly) or matched placebo via subcutaneous injection. Lipid changes during follow-up were analyzed.
Results
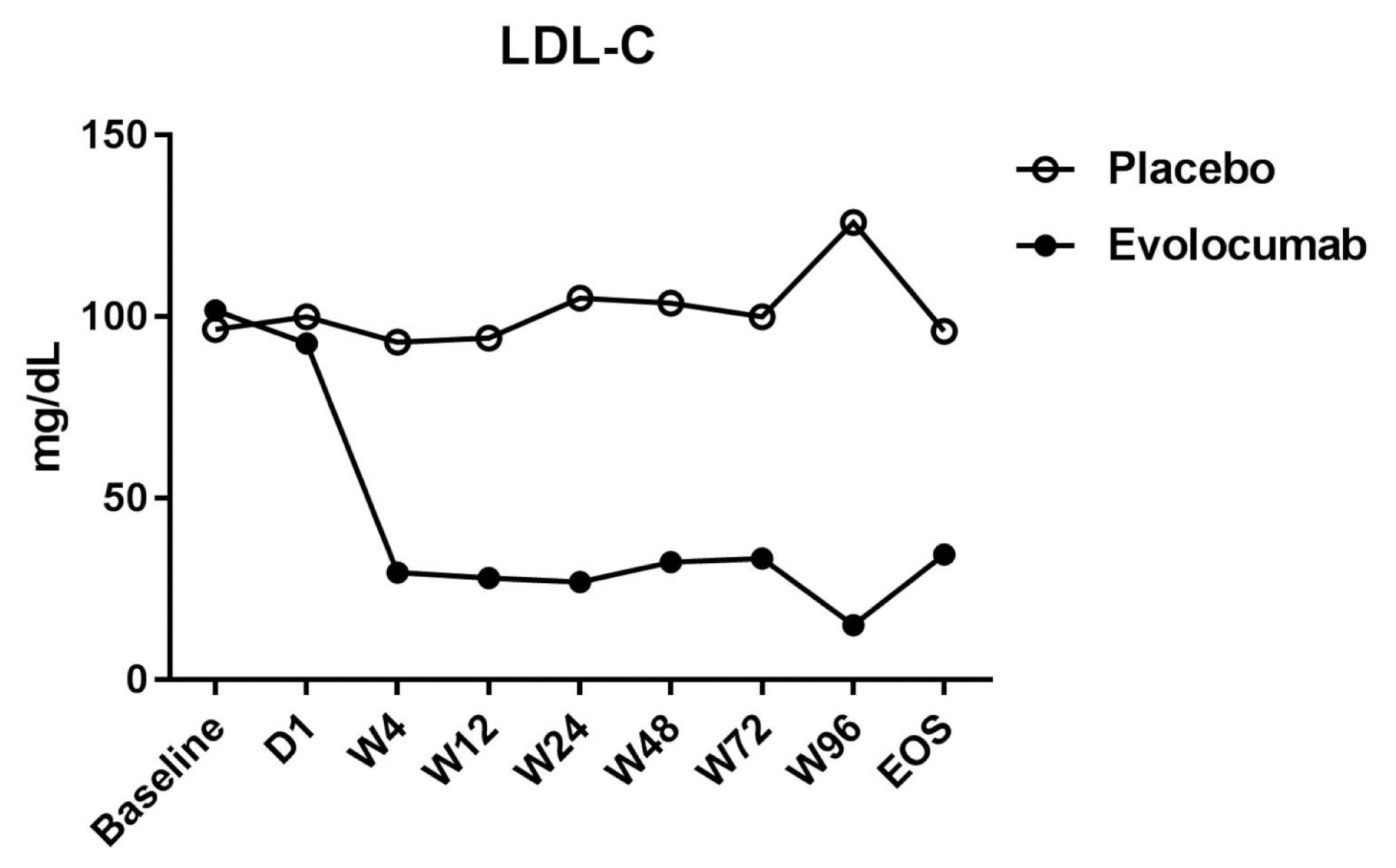
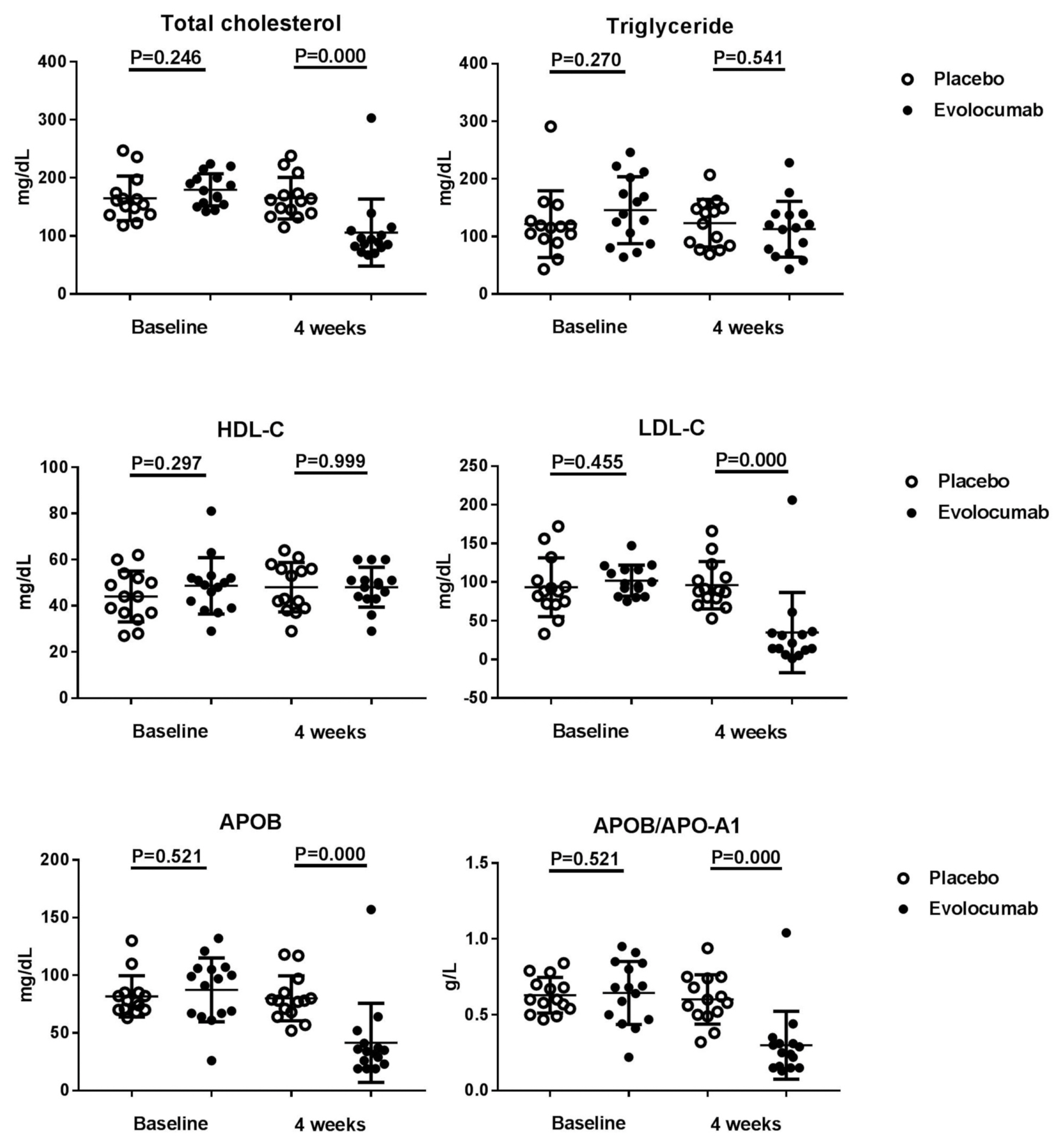
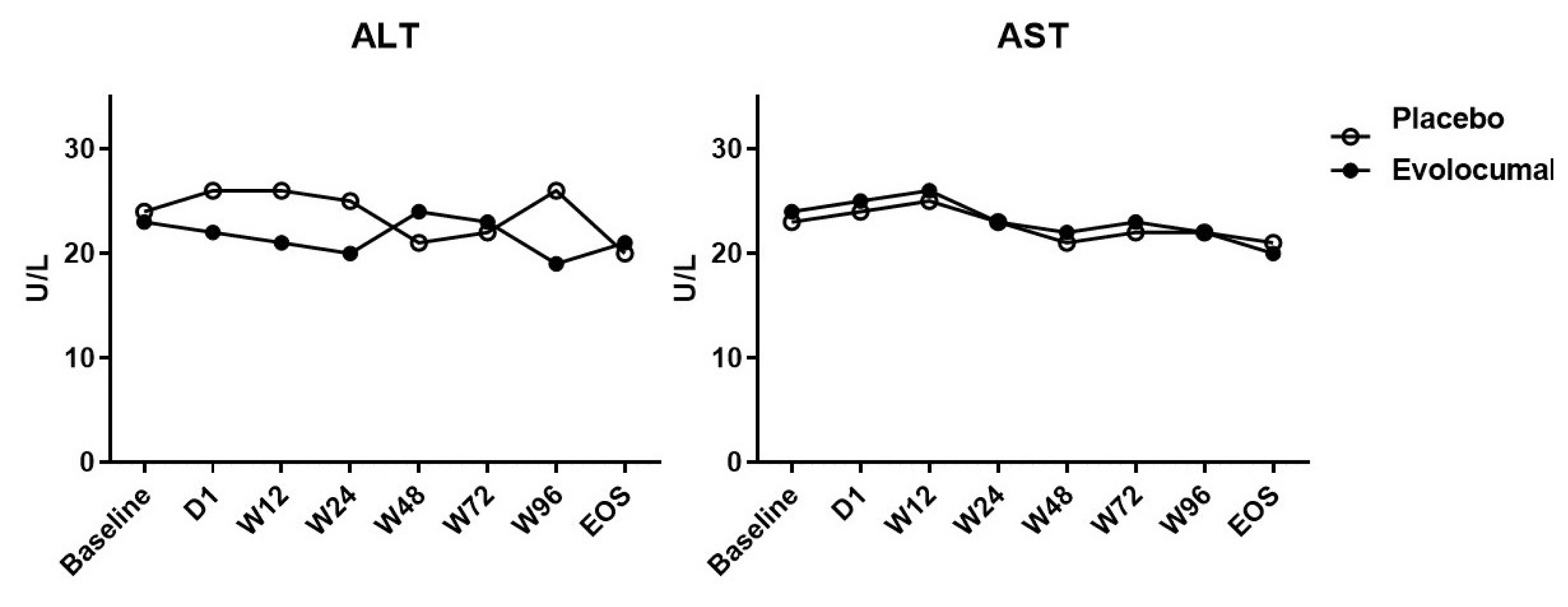
The median LDL cholesterol level at baseline was 88 mg per deciliter, and the average LDL cholesterol level was 101.8 ± 20.0 mg per deciliter. At 4 weeks, the median LDL cholesterol level was 39 mg per deciliter, and the average LDL cholesterol level was 34.8 ± 51.8 mg per deciliter. Compared to placebo group, the LDL cholesterol levels were significantly reduced after treatment (P < 0.001), as well as total cholesterol, ApoB, and ApoB / ApoA1 levels. During follow-up, no discomfort was reported at local injection sites, and no cases of abnormal liver function were observed.
Conclusions
Evolocumab significantly reduced LDL cholesterol levels and was well tolerated.
Figure
Reference
-
1. Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003; 100:928–33.
Article2. Abifadel M, Guerin M, Benjannet S, Rabès JP, Le Goff W, Julia Z, et al. Identification and characterization of new gain-of-function mutations in the PCSK9 gene responsible for autosomal dominant hypercholesterolemia. Atherosclerosis. 2012; 223:394–400.
Article3. Elagoz A, Benjannet S, Mammarbassi A, Wickham L, Seidah NG. Biosynthesis and Cellular Trafficking of the Convertase SKI-1/S1P Ectodomain Shedding Requires SKI-1 Activity. J Biol Chem. 2002; 277:11265–75.4. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol. 2015; 65:2638–51.
Article5. Rousselet E, Marcinkiewicz J, Kriz J, Zhou A, Hatten ME, Prat A, et al. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J Lipid Res. 2011; 52:1383–91.
Article6. Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003; 34:154–6.
Article7. Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005; 37:161–5.
Article8. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006; 354:1264–72.9. Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis. 2007; 193:445–8.
Article10. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014; 370:1809–19.
Article11. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, et al. Effect of evolocumab or ezetimibe added to moderate-or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. Jama. 2014; 311:1870–83.12. Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014; 63:2531–40.13. Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014; 63:2541–8.14. Raal FJ, Stein EA, Dufour R, Turmer T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015; 385:331–40.
Article15. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017; 376:1713–22.
Article16. Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. Jama. 2012; 308:2497–506.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of single-dose evolocumab injection in early-phase acute myocardial infarction: a retrospective single-center study
- Role of PCSK9 Inhibitors in Patients with Familial Hypercholesterolemia
- Efficacy of Diet Therapy in Korea Hypercholesterolemic Patients
- Effect of Lovastatin on Serum Lipids in Primary Hypercholesterolemia
- Treatment of Hypercholesterolemia in Elderly Patients; From the Viewpoint of Statins