Blood Res.
2020 Dec;55(4):262-274. 10.5045/br.2020.2020220.
Long-term treatment outcomes of children and adolescents with lymphoblastic lymphoma treated with various regimens:a single-center analysis
- Affiliations
-
- 1Department of Pediatrics, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea
- 2Division of Pediatric Hematology/Oncology, Department of Pediatrics, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea
- KMID: 2509965
- DOI: http://doi.org/10.5045/br.2020.2020220
Abstract
- Background
Lymphoblastic lymphoma (LBL) is the second most common subtype of pediatric non-Hodgkin lymphoma. Modified treatments derived from the LSA2-L2 regimen resulted in encouraging survival, but toxicities and long-term sequelae have been problematic. At present, the acute lymphoblastic leukemia (ALL)-type protocol has demonstrated efficacy in LBL. We analyzed the outcomes of children and adolescents with LBL treated with various regimens.
Methods
From 1991‒2018, this study enrolled 63 patients diagnosed with LBL at Asan Medical Center. Medical records were retrospectively analyzed.
Results
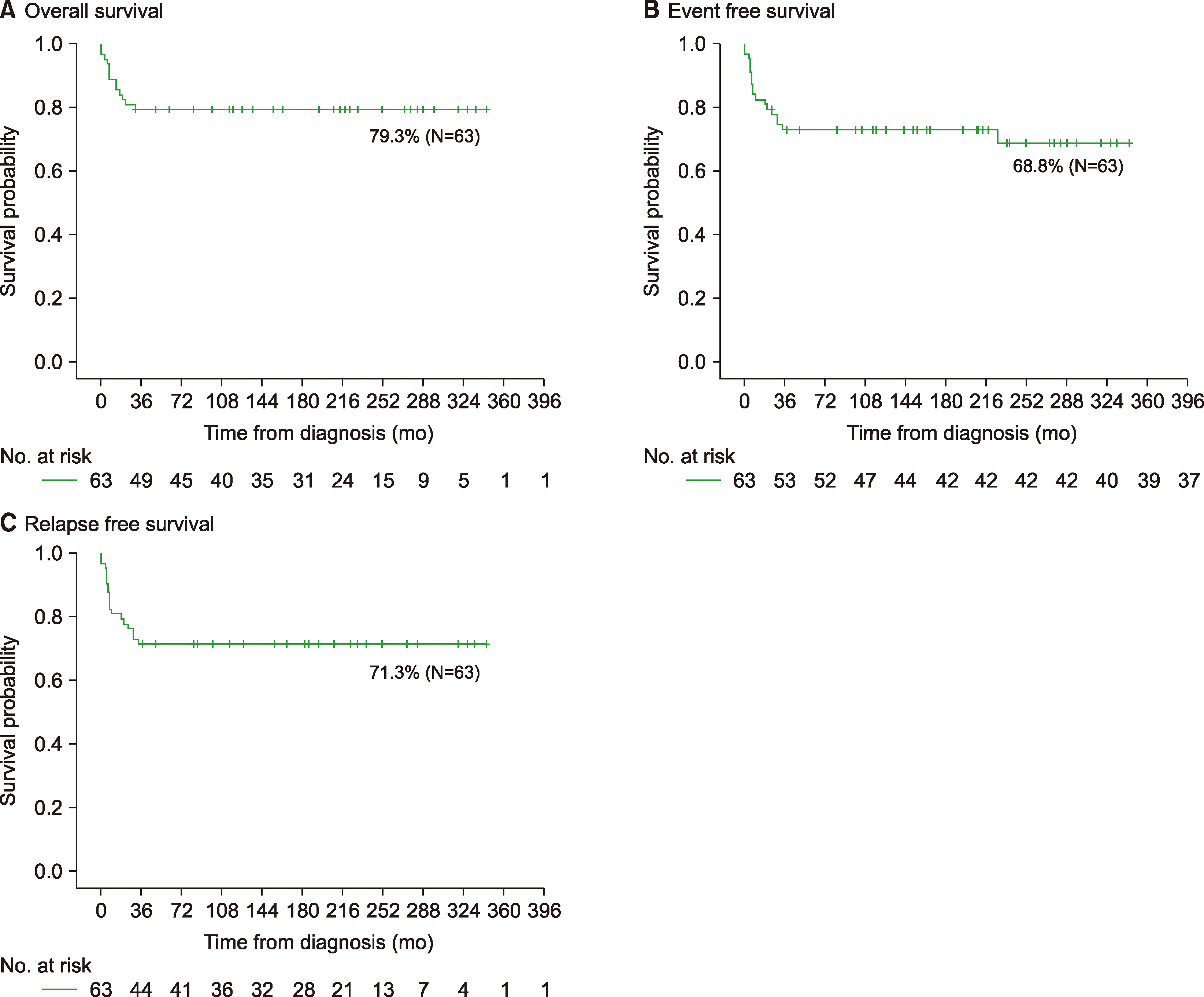
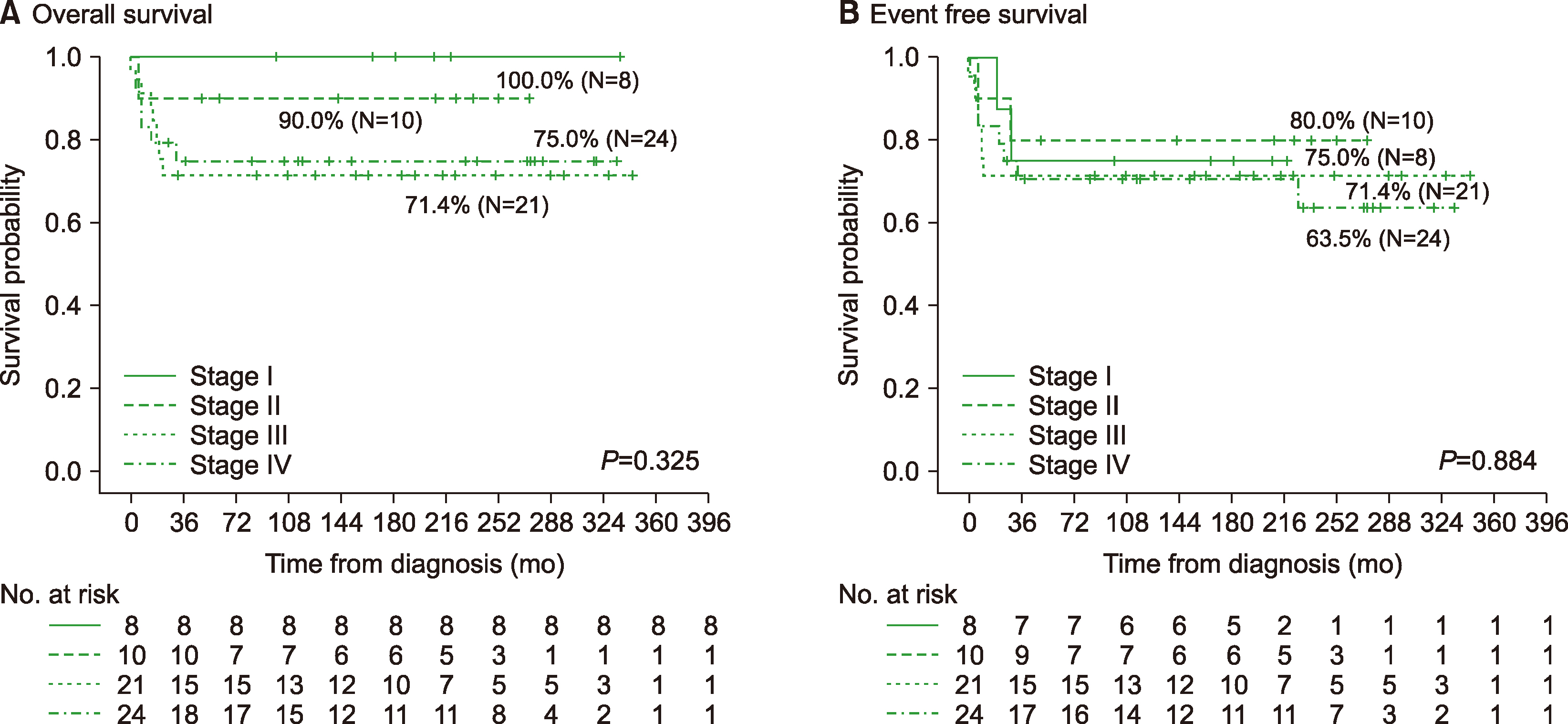
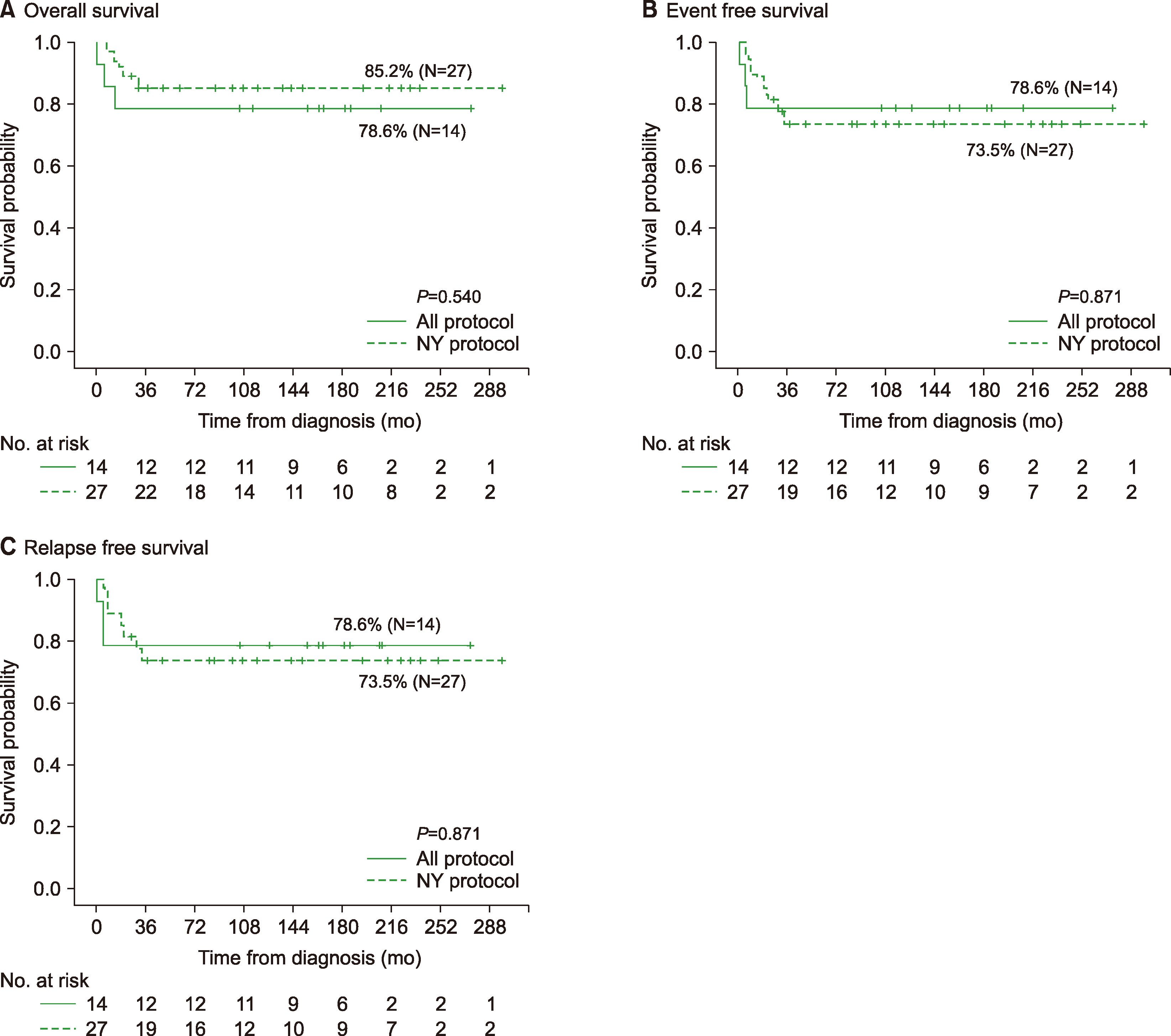
Among 63 patients, most patients (38.1%) presented with stage IV at diagnosis, and two had central nervous system (CNS) involvement. At a median follow-up of 160 months, the 5-year event free survival (EFS), overall survival (OS), and relapse free survival (RFS) were 68.8%, 79.3%, and 71.3%, respectively. Among 61 patients who received chemotherapy, 27 patients (44.3%) received the NY protocol, and 14 (23.0%) received the ALL-type protocol. There was no significant difference in 5-yr OS (85.2%/78.6%), EFS (73.5%/78.6%), and RFS (73.5%/78.6%) between the NY and ALL protocol groups, regardless of immunophenotype. Thirteen patients (21.3%) received prophylactic cranial radiotherapy with no difference in the incidence of CNS relapse based on irradiation.
Conclusion
This study showed no difference in outcome between the NY and ALL-type protocols, regardless of stage or immunophenotype. In addition to improving the effectiveness of treatment, it is necessary to continuously appraise the appropriate chemotherapy regimen, considering toxicities and long-term prognosis, for pediatric LBL.
Keyword
Figure
Reference
-
1. Reiter A, Schrappe M, Ludwig WD, et al. 2000; Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood. 95:416–21. PMID: 10627444.2. Anderson JR, Jenkin RD, Wilson JF, et al. 1993; Long-term follow-up of patients treated with COMP or LSA2L2 therapy for childhood non-Hodgkin's lymphoma: a report of CCG-551 from the Childrens Cancer Group. J Clin Oncol. 11:1024–32. DOI: 10.1200/JCO.1993.11.6.1024. PMID: 8501488.
Article3. Hvizdala EV, Berard C, Callihan T, et al. 1988; Lymphoblastic lymphoma in children--a randomized trial comparing LSA2-L2 with the A-COP+ therapeutic regimen: a Pediatric Oncology Group Study. J Clin Oncol. 6:26–33. DOI: 10.1200/JCO.1988.6.1.26. PMID: 3275750.
Article4. Lee SH, Yoo KH, Sung KW, Ko YH, Lee JW, Koo HH. 2013; Should children with non-Hodgkin lymphoma be treated with different protocols according to histopathologic subtype? Pediatr Blood Cancer. 60:1842–7. DOI: 10.1002/pbc.24695. PMID: 23857875.
Article5. Reiter A, Schrappe M, Parwaresch R, et al. 1995; Non-Hodgkin's lymphomas of childhood and adolescence: results of a treatment stratified for biologic subtypes and stage--a report of the Berlin- Frankfurt-Münster Group. J Clin Oncol. 13:359–72. DOI: 10.1200/JCO.1995.13.2.359. PMID: 7844597.6. Sekimizu M, Fujimoto J, Takimoto T, Tsurusawa M, Horibe K, Sunami S. 2018; Phase II clinical trial for newly diagnosed children and adolescents with localized lymphoblastic lymphoma (Japanese Leukemia/Lymphoma Study Group trial LLB-NHL03): study protocol for nationwide multicenter trial. Acta Med Okayama. 72:427–30. DOI: 10.18926/AMO/56183. PMID: 30140093.7. Song JS, Youn HS, Im HJ, Ghim T, Moon HN, Seo JJ. 2006; Treatment outcome and prognostic factors for children with advanced non-Hodgkin's lymphoma at a single institution. Korean J Hematol. 41:157–66. DOI: 10.5045/kjh.2006.41.3.157.
Article8. De Moerloose B, Suciu S, Bertrand Y, et al. 2010; Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): report of the EORTC randomized phase 3 trial 58951. Blood. 116:36–44. DOI: 10.1182/blood-2009-10-247965. PMID: 20407035. PMCID: PMC2904579.
Article9. Termuhlen AM, Smith LM, Perkins SL, et al. 2013; Disseminated lymphoblastic lymphoma in children and adolescents: results of the COG A5971 trial: a report from the Children's Oncology Group. Br J Haematol. 162:792–801. DOI: 10.1111/bjh.12460. PMID: 23889312.
Article10. Ochs J, Mulhern R, Fairclough D, et al. 1991; Comparison of neuropsychologic functioning and clinical indicators of neurotoxicity in long-term survivors of childhood leukemia given cranial radiation or parenteral methotrexate: a prospective study. J Clin Oncol. 9:145–51. DOI: 10.1200/JCO.1991.9.1.145. PMID: 1985164.
Article11. Clayton PE, Shalet SM, Morris-Jones PH, Price DA. 1988; Growth in children treated for acute lymphoblastic leukaemia. Lancet. 1:460–2. DOI: 10.1016/S0140-6736(88)91246-9. PMID: 2893877.
Article12. Schell MJ, Ochs JJ, Schriock EA, Carter M. 1992; A method of predicting adult height and obesity in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 10:128–33. DOI: 10.1200/JCO.1992.10.1.128. PMID: 1727914.
Article13. Mulhern RK, Fairclough D, Ochs J. 1991; A prospective comparison of neuropsychologic performance of children surviving leukemia who received 18-Gy, 24-Gy, or no cranial irradiation. J Clin Oncol. 9:1348–56. DOI: 10.1200/JCO.1991.9.8.1348. PMID: 2072138.
Article14. Bongers ME, Francken AB, Rouwé C, Kamps WA, Postma A. 2005; Reduction of adult height in childhood acute lymphoblastic leukemia survivors after prophylactic cranial irradiation. Pediatr Blood Cancer. 45:139–43. DOI: 10.1002/pbc.20334. PMID: 15714445.
Article15. Burkhardt B, Woessmann W, Zimmermann M, et al. 2006; Impact of cranial radiotherapy on central nervous system prophylaxis in children and adolescents with central nervous system-negative stage III or IV lymphoblastic lymphoma. J Clin Oncol. 24:491–9. DOI: 10.1200/JCO.2005.02.2707. PMID: 16421426.
Article16. Swerdlow SH, Campo E, Pileri SA, et al. 2016; The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 127:2375–90. DOI: 10.1182/blood-2016-01-643569. PMID: 26980727. PMCID: PMC4874220.
Article17. Termuhlen AM, Smith LM, Perkins SL, et al. 2012; Outcome of newly diagnosed children and adolescents with localized lymphoblastic lymphoma treated on Children's Oncology Group trial A5971: a report from the Children's Oncology Group. Pediatr Blood Cancer. 59:1229–33. DOI: 10.1002/pbc.24149. PMID: 22488718.
Article18. Hoelzer D, Gökbuget N, Digel W, et al. 2002; Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood. 99:4379–85. DOI: 10.1182/blood-2002-01-0110. PMID: 12036865.
Article19. Rhee ES, Kim H, Kang SH, et al. 2018; Outcome and prognostic factors in pediatric precursor t-cell acute lymphoblastic leukemia: a single-center experience. Clin Pediatr Hematol Oncol. 25:116–27. DOI: 10.15264/cpho.2018.25.2.116.
Article20. Schütt P, Passon J, Ebeling P, et al. 2007; Ifosfamide, etoposide, cytarabine, and dexamethasone as salvage treatment followed by high-dose cyclophosphamide, melphalan, and etoposide with autologous peripheral blood stem cell transplantation for relapsed or refractory lymphomas. Eur J Haematol. 78:93–101. DOI: 10.1111/j.1600-0609.2006.00796.x. PMID: 17313557.
Article21. Kobrinsky NL, Sposto R, Shah NR, et al. 2001; Outcomes of treatment of children and adolescents with recurrent non-Hodgkin's lymphoma and Hodgkin's disease with dexamethasone, etoposide, cisplatin, cytarabine, and l-asparaginase, maintenance chemotherapy, and transplantation: Children's Cancer Group Study CCG-5912. J Clin Oncol. 19:2390–6. DOI: 10.1200/JCO.2001.19.9.2390. PMID: 11331317.
Article22. Anderson JR, Wilson JF, Jenkin DT, et al. 1983; Childhood non- Hodgkin's lymphoma. The results of a randomized therapeutic trial comparing a 4-drug regimen (COMP) with a 10-drug regimen (LSA2-L2). N Engl J Med. 308:559–65. DOI: 10.1056/NEJM198303103081003. PMID: 6338381.23. Kim H, Seo H, Park Y, et al. 2018; APEX1 polymorphism and mercaptopurine-related early onset neutropenia in pediatric acute lymphoblastic leukemia. Cancer Res Treat. 50:823–34. DOI: 10.4143/crt.2017.351. PMID: 28882023. PMCID: PMC6056975.
Article24. Swerdlow SH, Campo E, Harris NL, editors. 2008. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. IARC Press;Lyon, France:25. Büchner T, Hiddemann W, Wörmann B, Schellong G, Ritter J, Creutzig U. 2001. Acute leukemias VIII: prognostic factors and treatment strategies. Springer-Verlag Berlin Heidelberg;Heidelberg, Germany: DOI: 10.1007/978-3-642-18156-6.26. Steinherz PG, Gaynon P, Miller DR, et al. 1986; Improved disease-free survival of children with acute lymphoblastic leukemia at high risk for early relapse with the New York regimen--a new intensive therapy protocol: a report from the Childrens Cancer Study Group. J Clin Oncol. 4:744–52. DOI: 10.1200/JCO.1986.4.5.744. PMID: 3517244.
Article27. Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. 2005; Cardiotoxicity of cancer therapy. J Clin Oncol. 23:7685–96. DOI: 10.1200/JCO.2005.08.789. PMID: 16234530.
Article28. Abromowitch M, Termuhlen A, Chang M, et al. 2008; High-dose methotrexate and early intensification of therapy do not improve 3 year EFS in children and adolescents with disseminated lymphoblastic lymphoma. Results of the randomized arms of COG A5971. Blood (ASH Annual Meeting Abstracts). 112(Suppl):3610. DOI: 10.1182/blood.V112.11.3610.3610.
Article29. Coustan-Smith E, Sandlund JT, Perkins SL, et al. 2009; Minimal disseminated disease in childhood T-cell lymphoblastic lymphoma: a report from the children's oncology group. J Clin Oncol. 27:3533–9. DOI: 10.1200/JCO.2008.21.1318. PMID: 19546402. PMCID: PMC2717759.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adolescents and young adults (AYA) with acute lymphoblastic leukemia
- Optimal therapy for adolescents and young adults with acute lymphoblastic leukemia-current perspectives
- Long Term Clinical Outcomes of Gastric Mucosa-Associated Lymphoid Tissue Lymphoma: A Single Center Study
- Clinical characteristics and treatment outcomes of children and adolescents with aggressive mature B-cell lymphoma: a single-center analysis
- Long-Term Survival after T-cell Lymphoblastic Lymphoma Treated with One Cycle of Hyper-CVAD Regimen