Blood Res.
2020 Dec;55(4):253-261. 10.5045/br.2020.2020070.
First report of the unique expression of RECAF (receptor for alfa feto-protein) in adult B-NHL/CLL patients
- Affiliations
-
- 1Clinical Pathology Department, Internal Medicine Department, Ain Shams University, Faculty of Medicine, Cairo, Egypt
- 2Clinical Hematology and Bone Marrow Transplant Unit, Internal Medicine Department, Ain Shams University, Faculty of Medicine, Cairo, Egypt
- KMID: 2509964
- DOI: http://doi.org/10.5045/br.2020.2020070
Abstract
- Background
Lymphoproliferative disorders (LPDs) are a heterogeneous group of diseases characterized by an uncontrolled production of monoclonal lymphocytes. RECAF is the receptor for alpha-fetoprotein, which is re-expressed on malignant cells, thus serving as a broad-spectrum tumor marker.
Methods
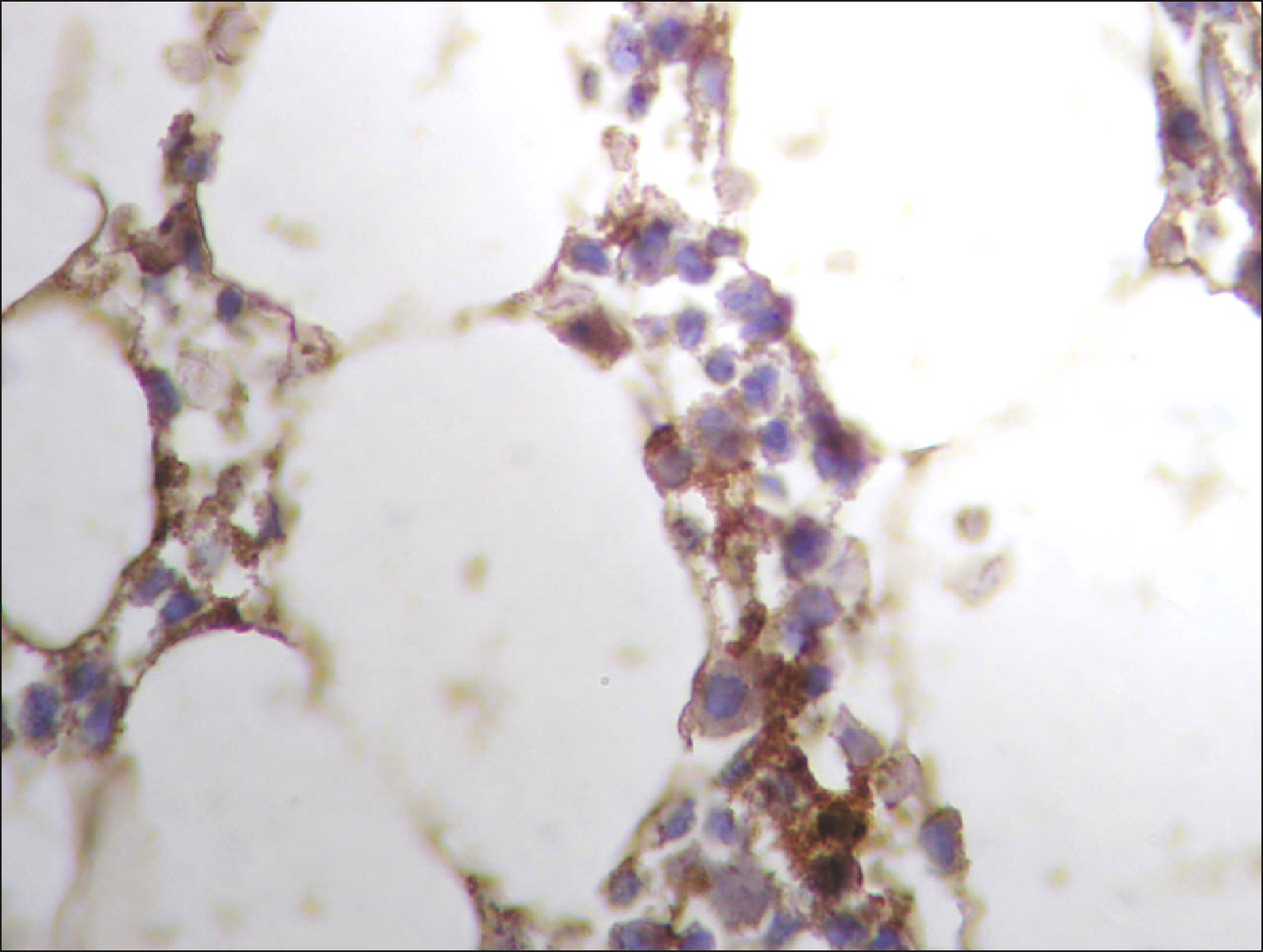
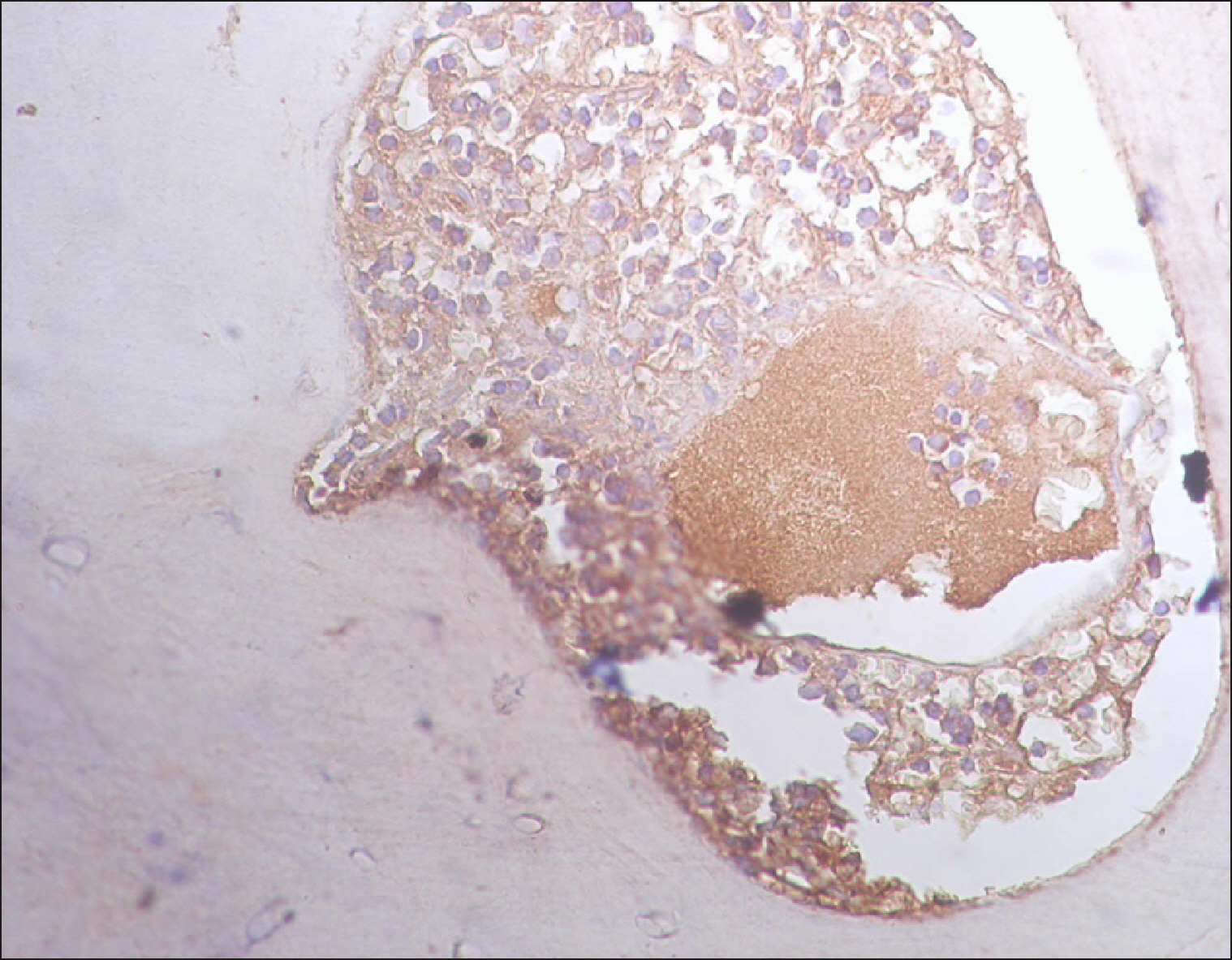
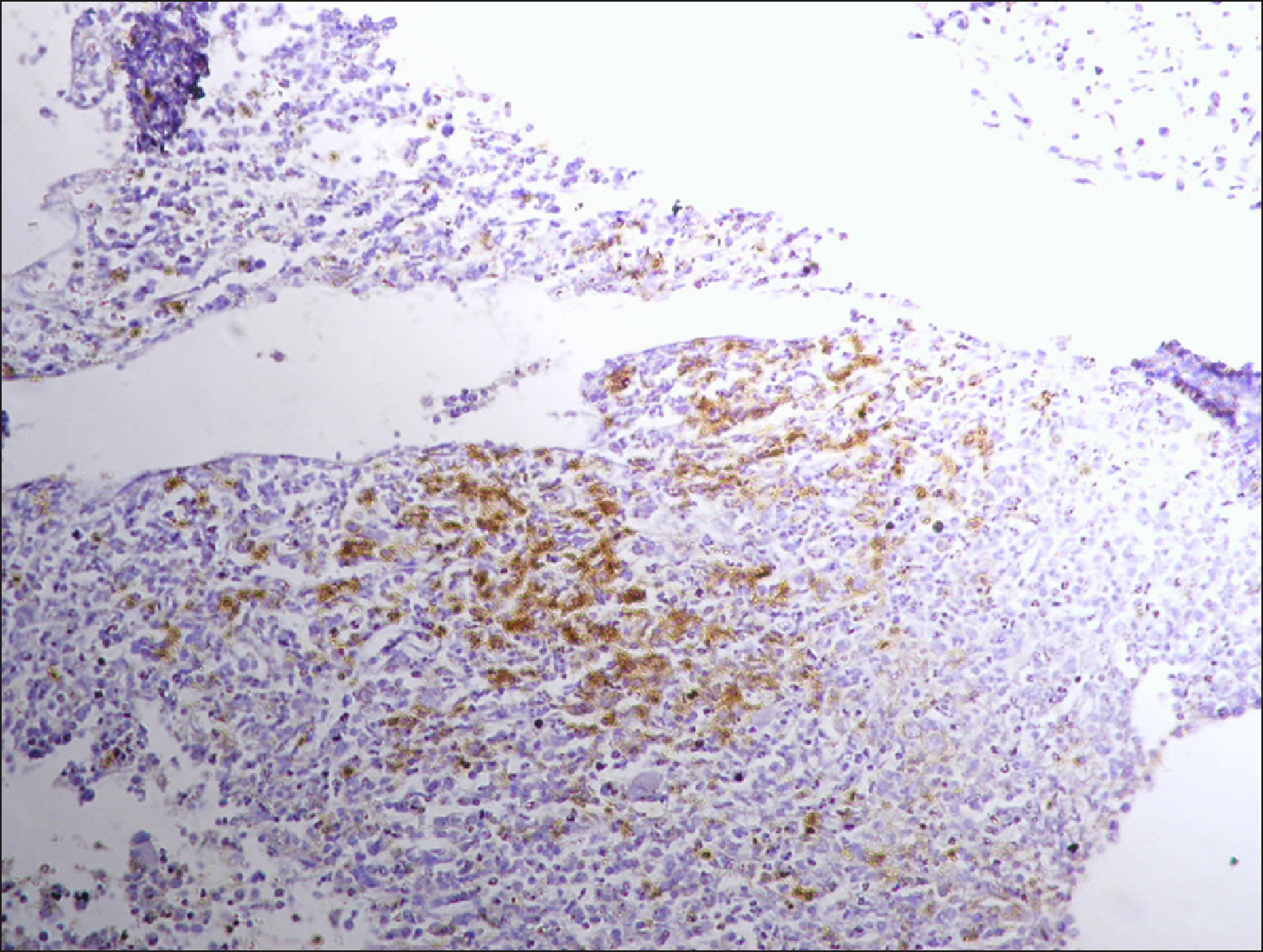
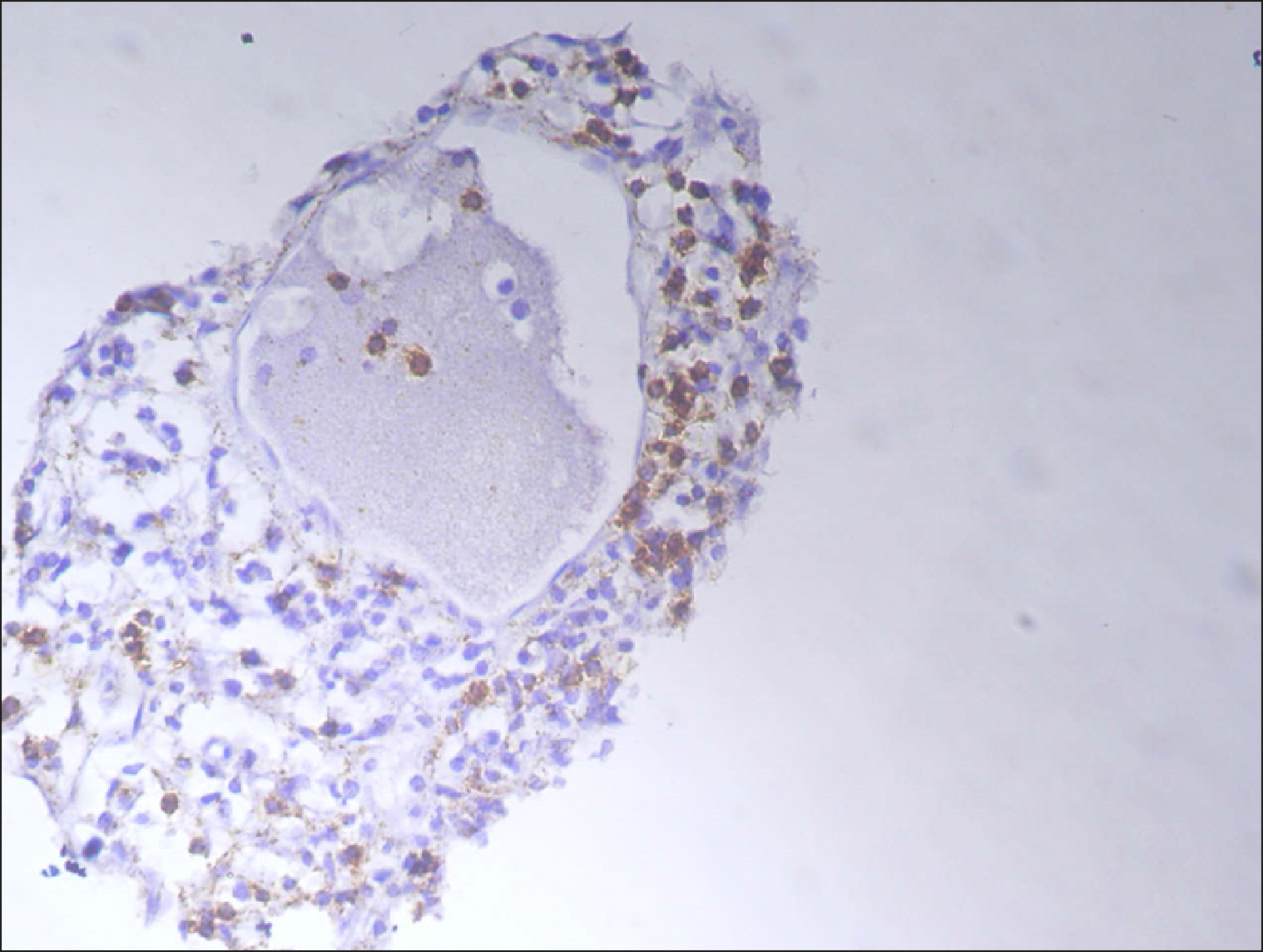
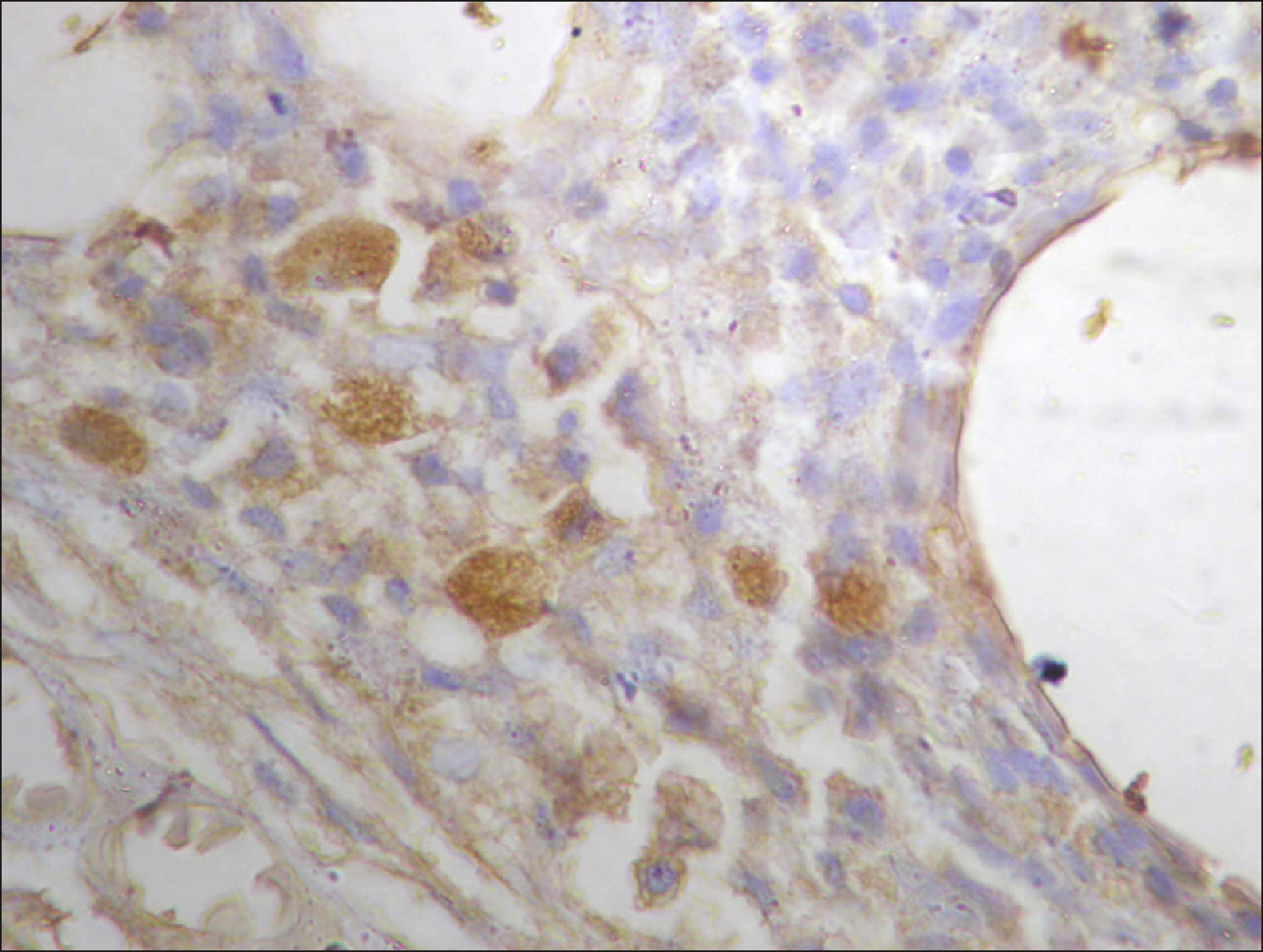
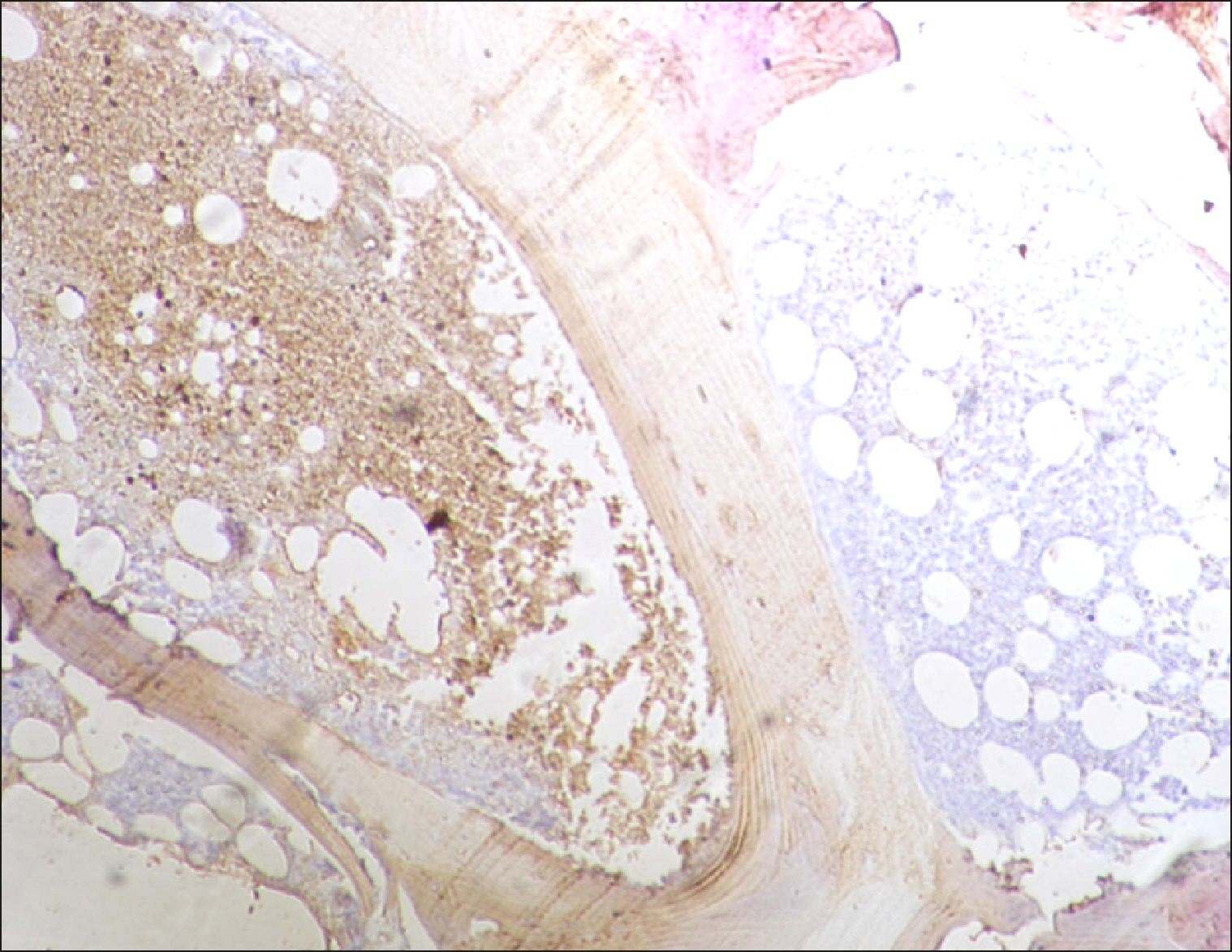
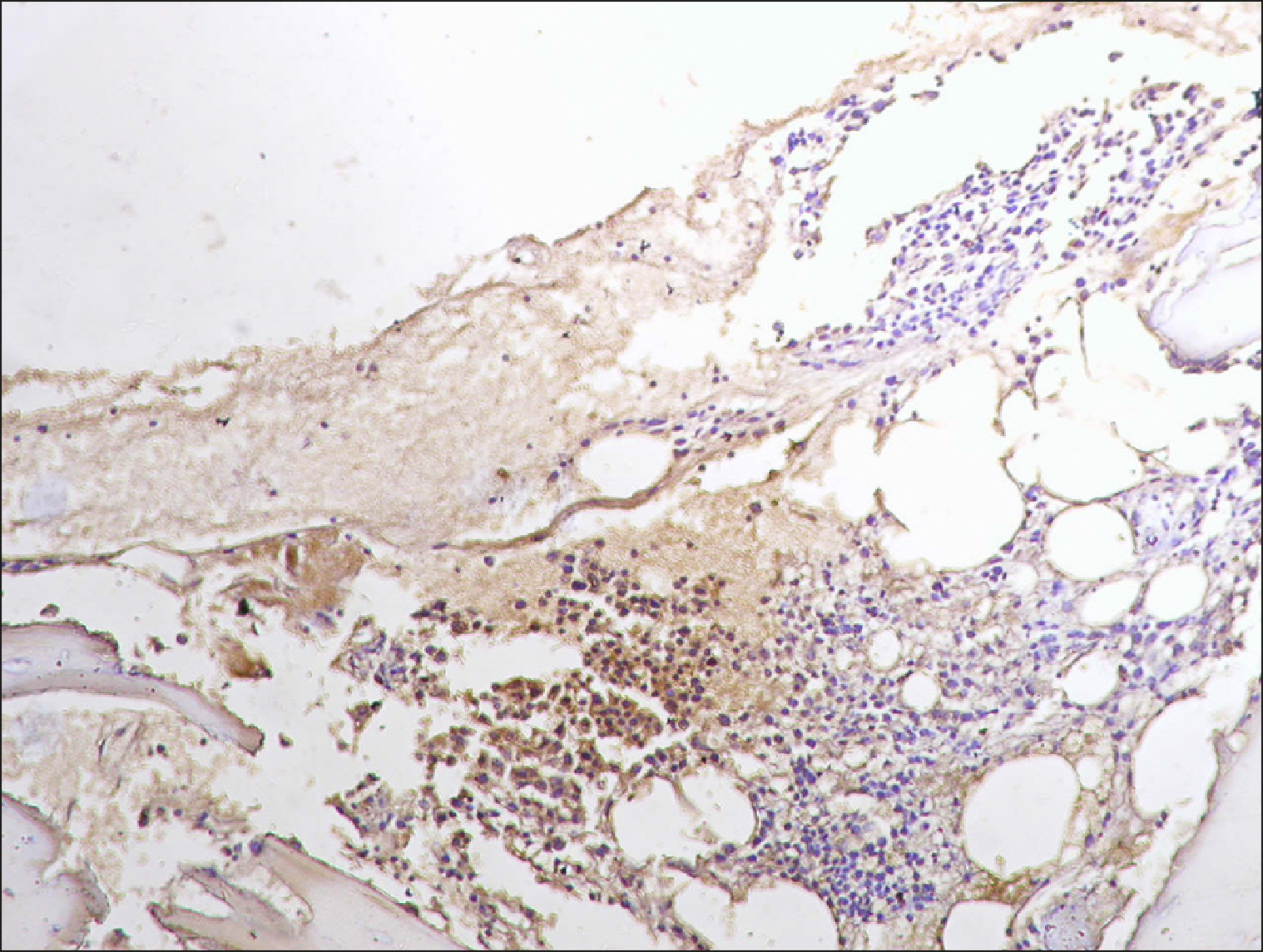
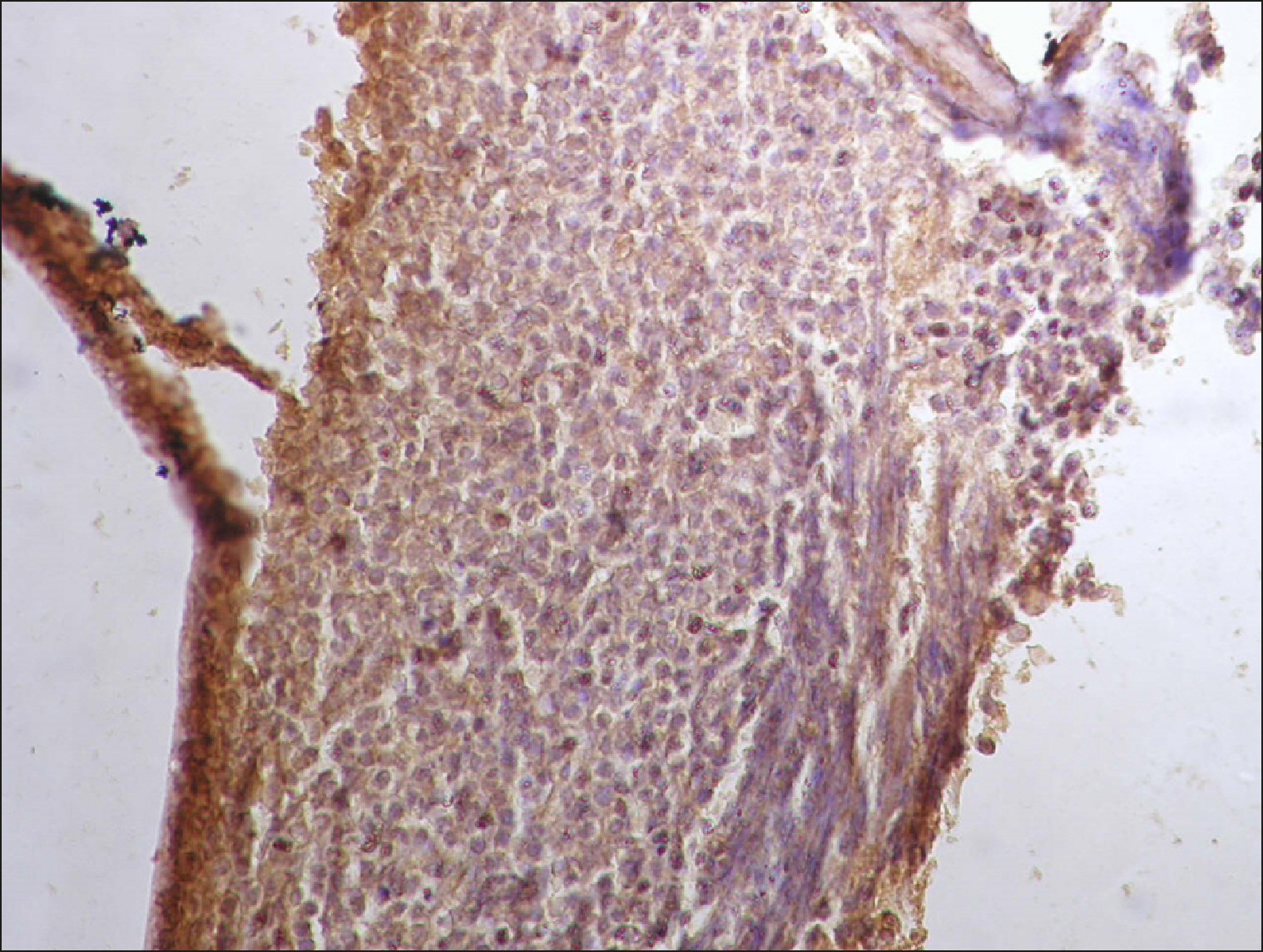
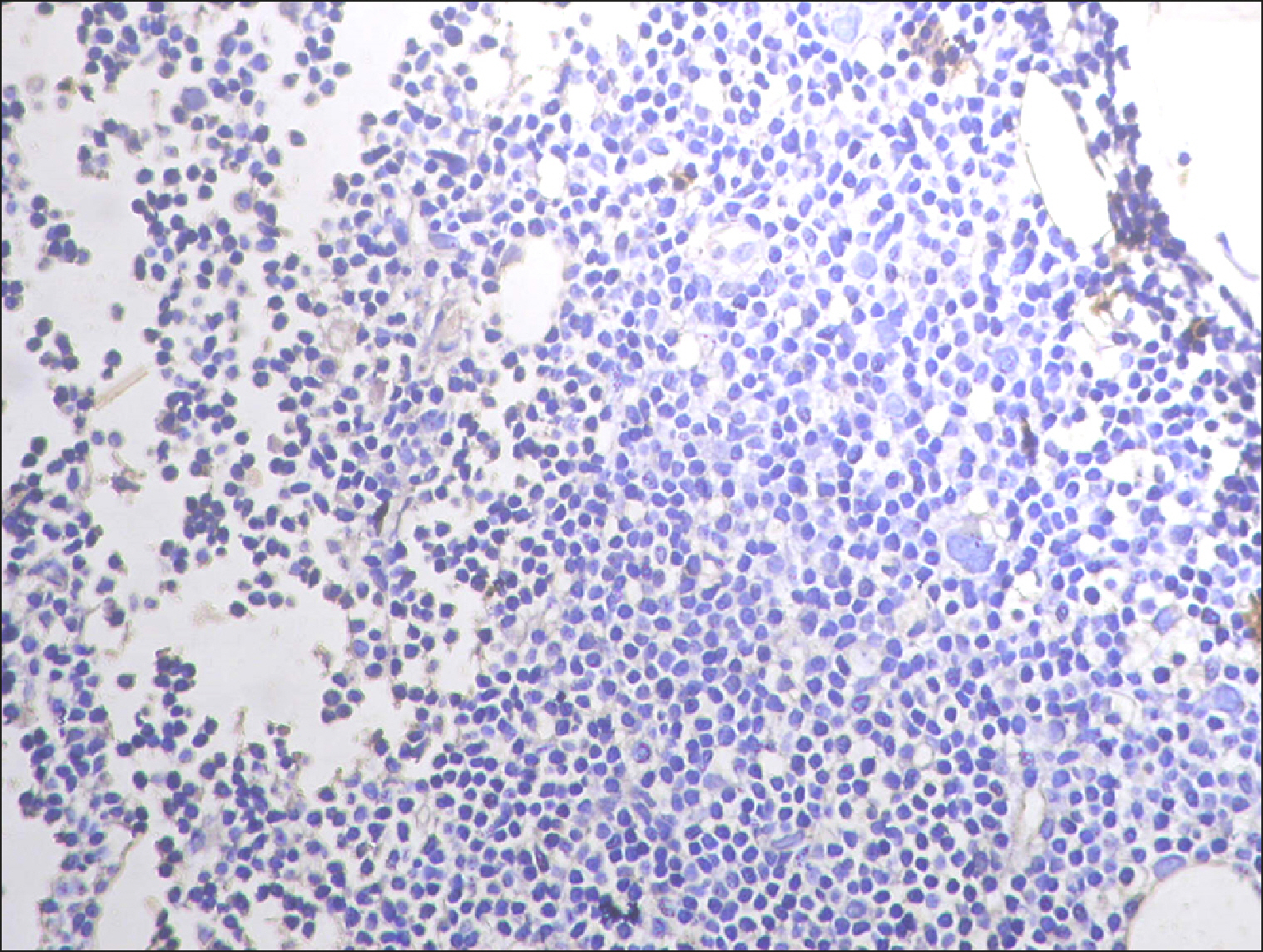
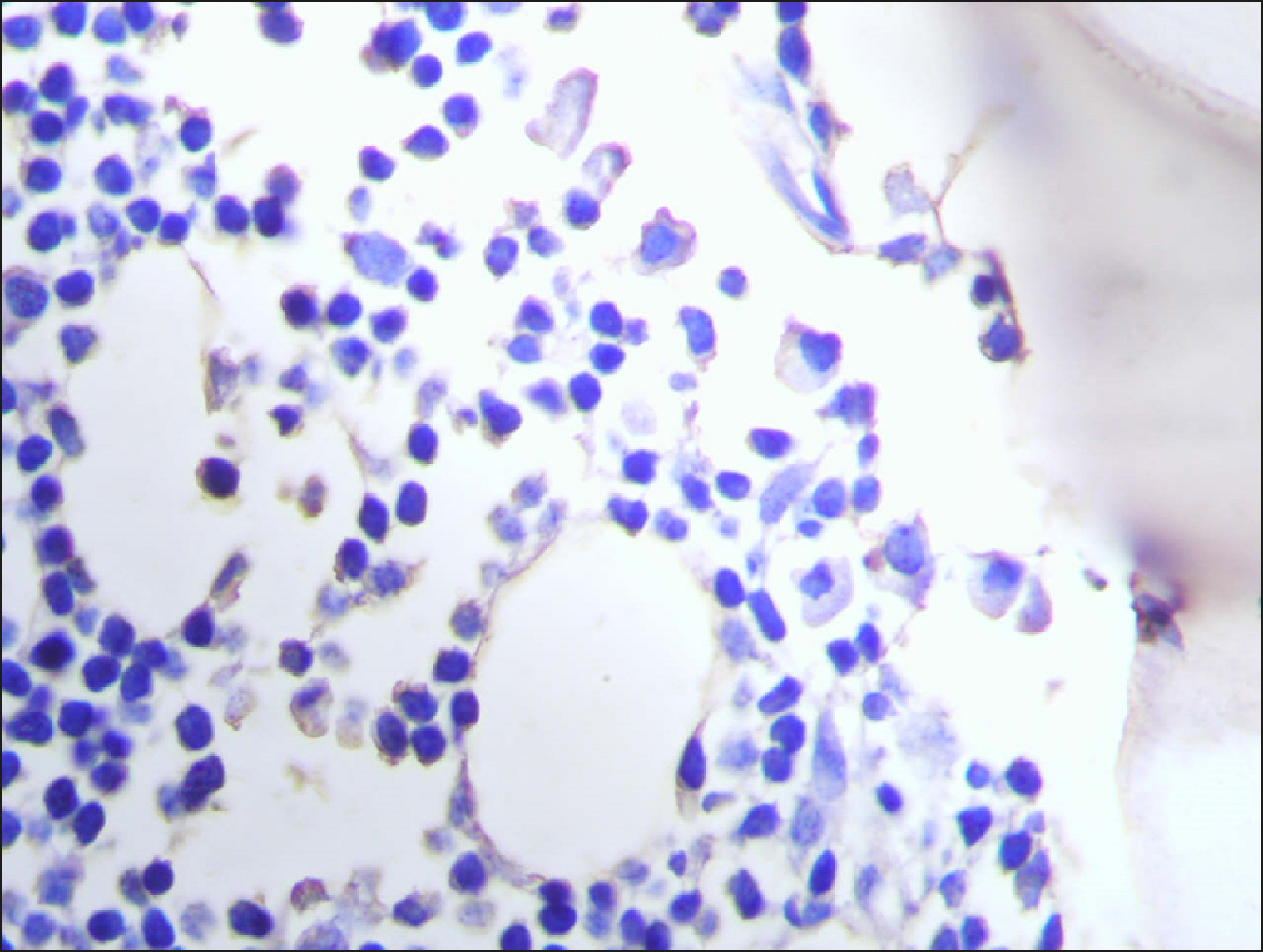
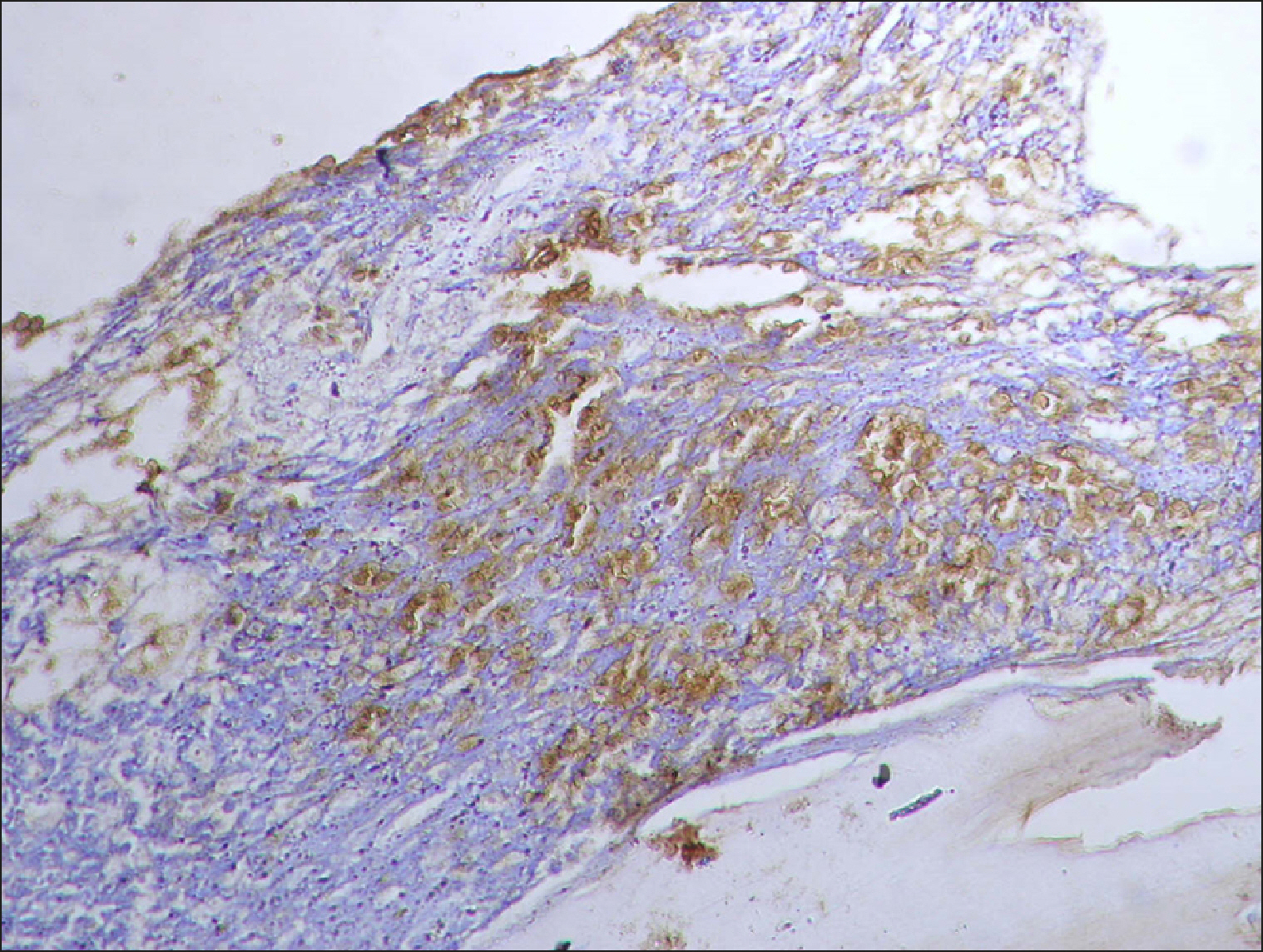
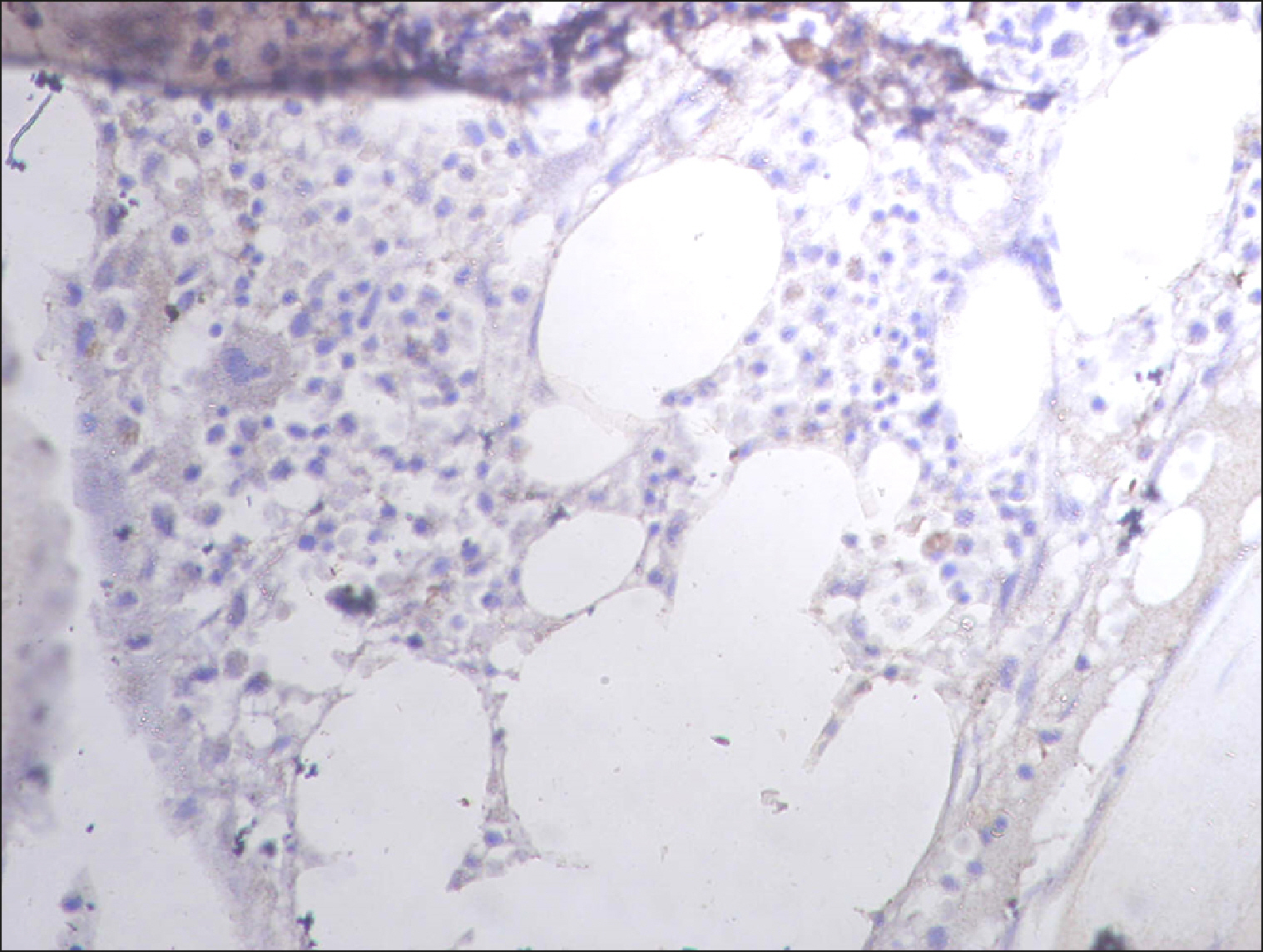
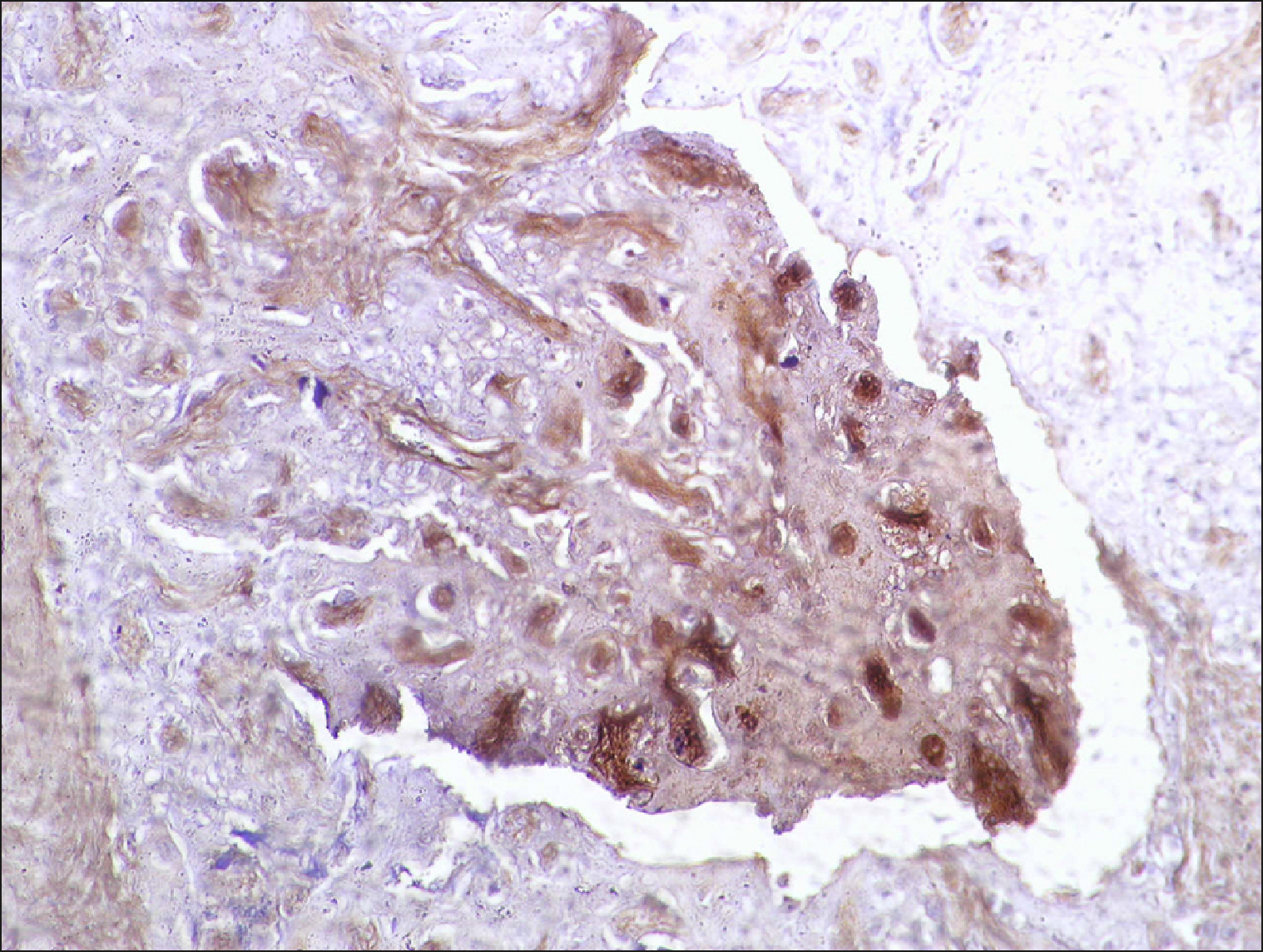
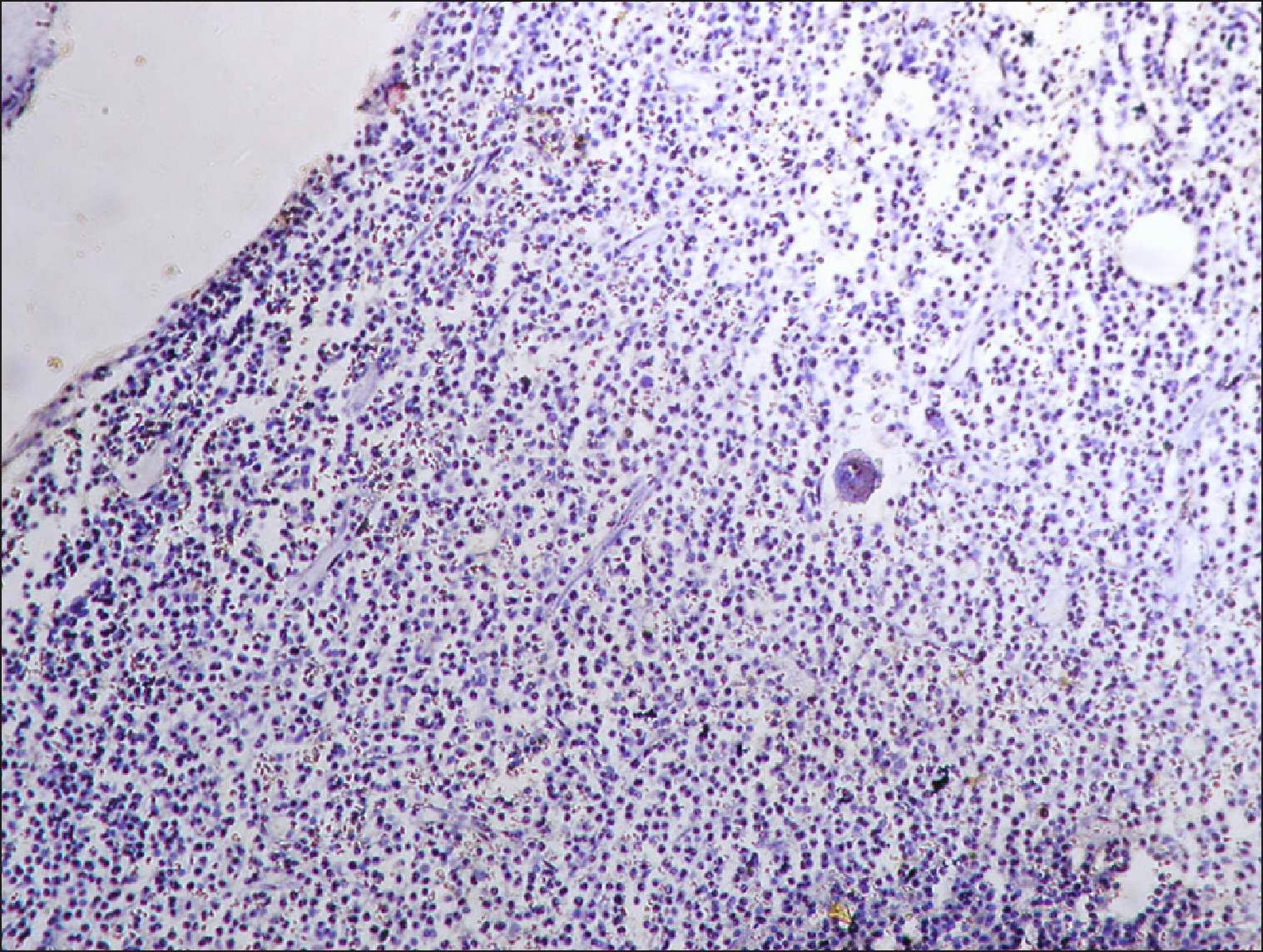
The current study is a retrospective study carried out on 200 archival bone marrow trephine biopsy specimens [60 normal control (NC), 38 pathological control (PC) and 102 lymphoproliferative diseases (LPD) specimens]. RECAF expression was assessed using immunohistochemistry.
Results
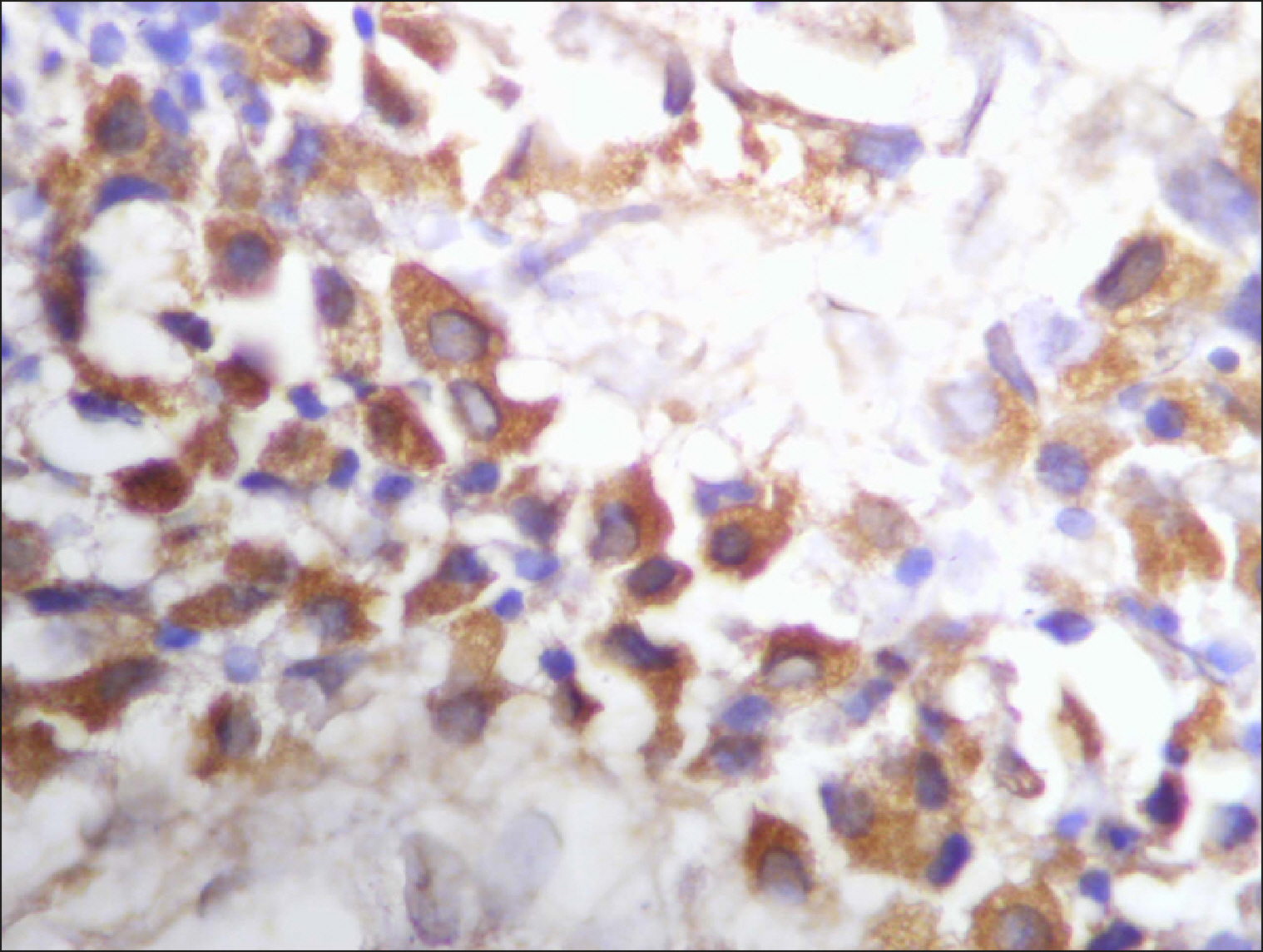
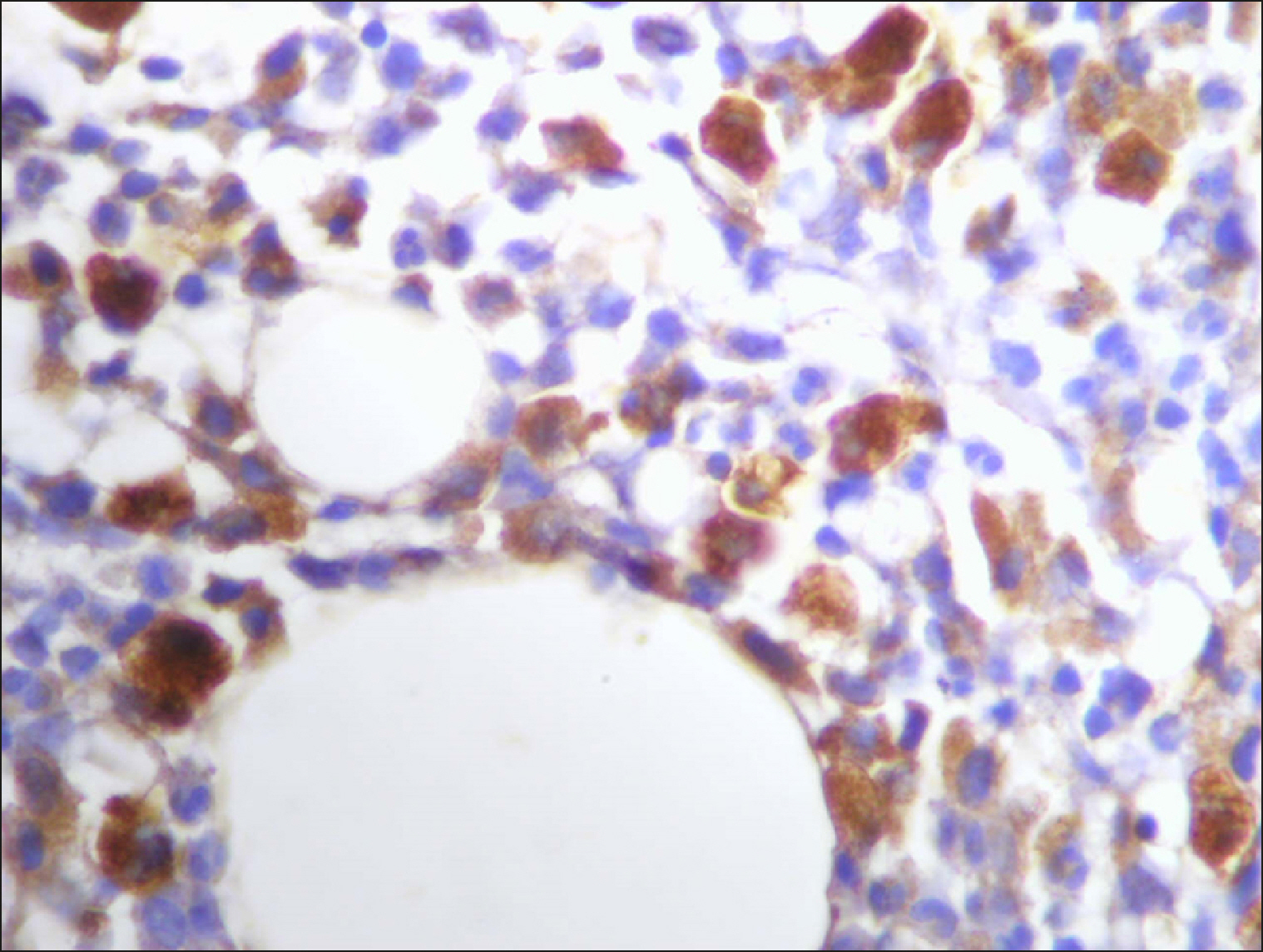
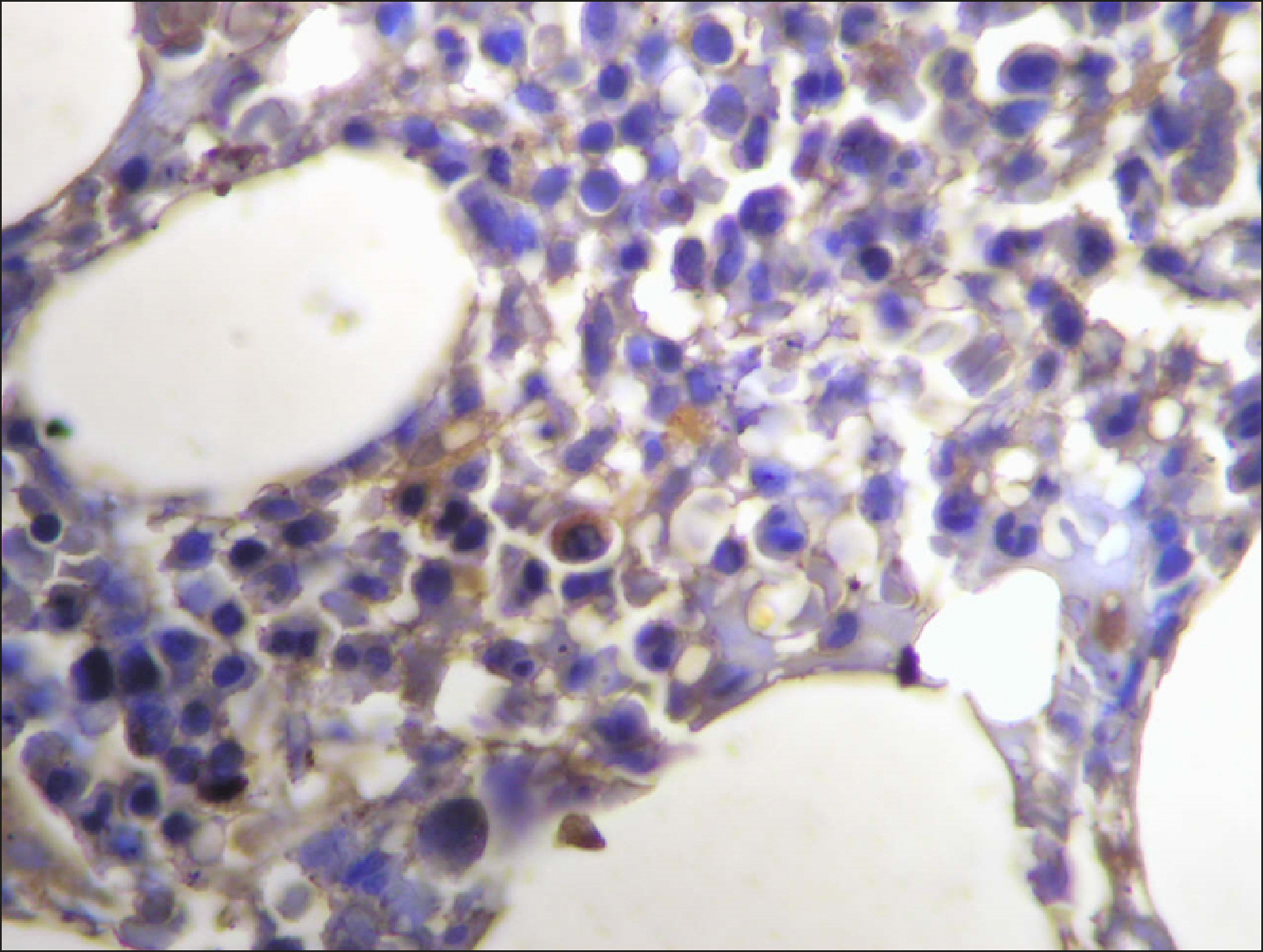
The percentage of cells that are positive for RECAF was significantly higher in the LPD group than in the NC group (P=0.007), while there was no significant difference between non-Hodgkin lymphoma (NHL) patients and PC regarding the number of RECAF positive cells (P=0.1). RECAF showed a unique expression pattern among the different subtypes of LPD. None of the hairy cell leukemia (HCL) expressed RECAF, while the highest percentage was seen in follicular lymphoma (FL) and diffuse large B cell lymphoma (DLBCL) (P=0.001). Compared to routine histopathology, RECAF was more sensitive in detecting bone marrow (BM) infiltration in FL, mantle cell lymphoma (MCL), and DLBCL (P=0.01).
Conclusion
RECAF is significantly expressed in the BM of NHL/chronic lymphocytic leukemia (CLL) patients. RECAF shows a unique expression pattern among the different subtypes of LPD. Furthermore, RECAF may help to detect bone marrow infiltration in lymphoma cells. This may help in the diagnosis, follow-up, and targeting of LPD.
Keyword
Figure
Reference
-
1. Swerdlow SH, Campo E, Pileri SA, et al. 2016; The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 12:2375–90. DOI: 10.1182/blood-2016-01-643569. PMID: 26980727. PMCID: PMC4874220.
Article2. Park Y, Park BB, Jeong JY, et al. 2016; Assessment of bone marrow involvement in patients with lymphoma: report on a consensus meeting of the Korean Society of Hematology Lymphoma Working Party. Korean J Intern Med. 31:1030–41. DOI: 10.3904/kjim.2015.006. PMID: 27809449. PMCID: PMC5094919.
Article3. Alpert E, Feller ER. 1978; Alpha-fetoprotein (AFP) in benign liver disease. Evidence that normal liver regeneration does not induce AFP synthesis. Gastroenterology. 74(5 Pt 1):856–8. DOI: 10.1016/0016-5085(78)90141-5. PMID: 76587.4. Deutsch HF. 1991; Chemistry and biology of alpha-fetoprotein. Adv Cancer Res. 56:253–312. DOI: 10.1016/S0065-230X(08)60483-2. PMID: 1709334.5. Moro R, Gulyaeva-Tcherkassova J, Stieber P. 2012; Increased alpha- fetoprotein receptor in the serum of patients with early-stage breast cancer. Curr Oncol. 19:e1–8. DOI: 10.3747//co.19.979. PMID: 22328843. PMCID: PMC3267592.
Article6. Geuskens M, Naval J, Uriel J. 1986; Ultrastructural studies of the intracellular translocation of endocytosed alpha-foetoprotein (AFP) by cytochemistry and of the uptake of 3H-arachidonic acid bound to AFP by autoradiography in rat rhabdomyosarcoma cells. J Cell Physiol. 128:389–96. DOI: 10.1002/jcp.1041280307. PMID: 2427529.7. Uriel J, Naval J, Laborda J. 1987; alpha-Fetoprotein-mediated transfer of arachidonic acid into cultured cloned cells derived from a rat rhabdomyosarcoma. J Biol Chem. 262:3579–85. PMID: 2434503.
Article8. Hajeri-Germond M, Naval J, Trojan J, Uriel J. 1985; The uptake of alpha-foetoprotein by C-1300 mouse neuroblastoma cells. Br J Cancer. 51:791–7. DOI: 10.1038/bjc.1985.123. PMID: 2408647. PMCID: PMC1977076.
Article9. Moro R, Fielitz W, Esteves A, Grunberg J, Uriel J. 1984; In vivo uptake of heterologous alphafetoprotein and serum albumin by ependymal cells of developing chick embryos. Int J Dev Neurosci. 2:143–8. DOI: 10.1016/0736-5748(84)90005-4. PMID: 24873957.
Article10. Moro R, Heuguerot C, Vercelli-Retta J, Fielitz W, López JJ, Roca R. 1984; The use of radioiodinated alpha-fetoprotein for the scintigraphic detection of mouse mammary carcinomas. Nucl Med Commun. 5:5–12. DOI: 10.1097/00006231-198401000-00002. PMID: 6085737.
Article11. Uriel J, Poupon MF, Geuskens M. 1983; Alphafoetoprotein uptake by cloned cell lines derived from a nickel-induced rat rhabdo-myosarcoma. Br J Cancer. 48:261–9. DOI: 10.1038/bjc.1983.181. PMID: 6192837. PMCID: PMC2011436.
Article12. Uriel J, Villacampa MJ, Moro R, Naval J, Failly-Crépin C. 1984; Uptake of radiolabeled alpha-fetoprotein by mouse mammary car-cinomas and its usefulness in tumor scintigraphy. Cancer Res. 44:5314–9. PMID: 6207915.13. Naval J, Villacampa MJ, Goguel AF, Uriel J. 1985; Cell-type-specific receptors for alpha-fetoprotein in a mouse T-lymphoma cell line. Proc Natl Acad Sci U S A. 82:3301–5. DOI: 10.1073/pnas.82.10.3301. PMID: 2582410. PMCID: PMC397763.
Article14. Villacampa MJ, Moro R, Naval J, Failly-Crepin C, Lampreave F, Uriel J. 1984; Alpha-fetoprotein receptors in a human breast cancer cell line. Biochem Biophys Res Commun. 122:1322–7. DOI: 10.1016/0006-291X(84)91236-1. PMID: 6206854.
Article15. Mizejewski GJ. 2002; Biological role of alpha-fetoprotein in cancer: prospects for anticancer therapy. Expert Rev Anticancer Ther. 2:709–35. DOI: 10.1586/14737140.2.6.709. PMID: 12503217.16. Benno RW, Williams TH. 1978; Evidence for intracellular localization of alpha-fetoprotein in the developing rat brain. Brain Res. 142:182–6. DOI: 10.1016/0006-8993(78)90189-0. PMID: 75046.17. Moro R, Uriel J. 1981; Early localization of alpha-fetoprotein in the developing nervous system of the chicken. Oncodev Biol Med. 2:391–8. PMID: 6180413.18. Uriel J, Trojan J, Dubouch P, Pineiro A. 1982; Intracellular alpha- fetoprotein and albumin in the developing nervous system of the baboon. Pathol Biol (Paris). 30:79–83. PMID: 6178072.19. Toran-Allerand CD. 1980; Coexistence of alpha-fetoprotein, albumin and transferrin immunoreactivity in neurones of the developing mouse brain. Nature. 286:733–5. DOI: 10.1038/286733a0. PMID: 6157991.20. Trojan J, Uriel J. 1982; Immunocytochemical localisation of alpha- fetoprotein (AFP) and serum albumin (ALB) in ecto-, meso- and endodermal tissue derivatives of the developing rat. Oncodev Biol Med. 3:13–22. PMID: 6181479.21. Lorenzo HC, Geuskens M, Macho A, et al. 1996; Alpha-fetoprotein binding and uptake by primary cultures of human skeletal muscle. Tumour Biol. 17:251–60. DOI: 10.1159/000217986. PMID: 8685605.
Article22. Uriel J, Failly-Crepin C, Villacampa MJ, Pineiro A, Geuskens M. 1984; Incorporation of alphafetoprotein by the MCF-7 human breast cancer cell line. Tumour Biol. 5:41–51. PMID: 6208595.23. Moro R, Tamaoki T, Wegmann TG, Longenecker BM, Laderoute MP. 1993; Monoclonal antibodies directed against a widespread oncofetal antigen: the alpha-fetoprotein receptor. Tumour Biol. 14:116–30. DOI: 10.1159/000217864. PMID: 7687070.
Article24. Laderoute M, Willans D, Wegmann T, Longenecker M. 1994; The identification, isolation and characterization of a 67 kilodalton, PNA-reactive autoantigen commonly expressed in human adenocarcinomas. Anticancer Res. 14:1233–45. PMID: 7520680.25. Alava MA, Iturralde M, Lampreave F, Piñeiro A. 1999; Specific uptake of alpha-fetoprotein and albumin by rat Morris 7777 hepatoma cells. Tumour Biol. 20:52–64. DOI: 10.1159/000056521. PMID: 9858875.
Article26. Mizejewski GJ, Mirowski M, Garnuszek P, et al. 2010; Targeted delivery of anti-cancer growth inhibitory peptides derived from human alpha-fetoprotein: review of an International Multi-Center Collaborative Study. J Drug Target. 18:575–88. DOI: 10.3109/10611861003587243. PMID: 20151941.27. Pak VN. 2018; Selective targeting of myeloid-derived suppressor cells in cancer patients through AFP-binding receptors. Future Sci OA. 5:FSO321. DOI: 10.4155/fsoa-2018-0029. PMID: 30652015. PMCID: PMC6331696.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal panel of immunohistochemistry for the diagnosis of B-cell non-Hodgkin lymphoma using bone marrow biopsy: a tertiary care center study
- Expression of TRAIL (Apo-2L)/TRAIL Receptor System Related to Apoptosis at the Human Extraembryonic Tissues and Gestational Trophoblastic Disease
- p53 protein expression and its prognostic importance in patients with nodal non-Hodgkin's lymphoma
- Smudge cell percentage as a surrogate marker for ZAP-70 expression in patients with chronic lymphocytic leukemia
- Immunohistochemical Study of Bcl-2 Oncoprotein Expression in Childhood Non-Hodgkin's Lymphoma