Korean Circ J.
2021 Jan;51(1):43-55. 10.4070/kcj.2020.0391.
An Open-label, Single-arm, Multicenter Feasibility Study Evaluating the Safety of Catheter-based Renal Denervation with DENEX™ in Patients with Uncontrolled Hypertension on Standard Medical Therapy
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Uijeongbu St. Mary's Hospital, The Catholic University of Korea College of Medicine, Uijeongbu, Korea
- 2Department of Cardiology, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea
- 3Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea
- 4Cardiovascular Center, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2509902
- DOI: http://doi.org/10.4070/kcj.2020.0391
Abstract
- Background and Objectives
DENEX™ is a novel renal sympathetic denervation (RDN) system that is equipped with 3 electrodes that deliver radiofrequency energy to the renal nerves along renal arteries. The purpose of this study was to evaluate the safety and efficacy of RDN with DENEX™ in resistant hypertension.
Methods
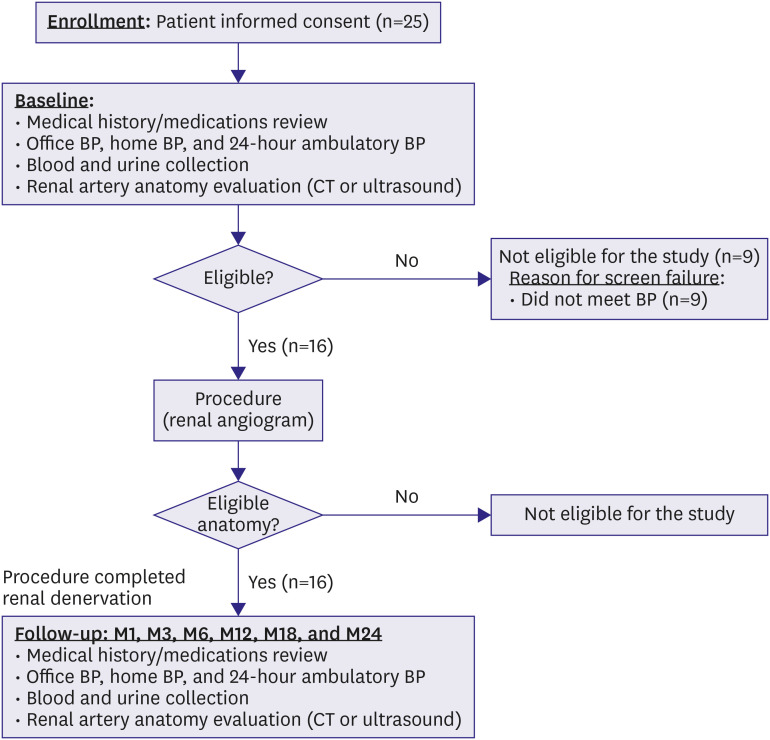
This was an open-label, single-arm, multicenter, first-in-man pilot study. Between November 2016 and May 2018, a total of 16 patients were enrolled at 4 centers in South Korea. The inclusion criteria were systolic blood pressure (SBP) ≥150 mmHg and use of 3 or more antihypertensive medications, including diuretics. The primary objective was the safety outcome of RDN with the DENEX™ system. The secondary objective was efficacy outcome based on changes of office, and 24-hour ambulatory SBP from baseline to 3 months. The patients underwent abdominal computed tomography (CT) or duplex ultrasonogram before and 6 months after RDN.
Results
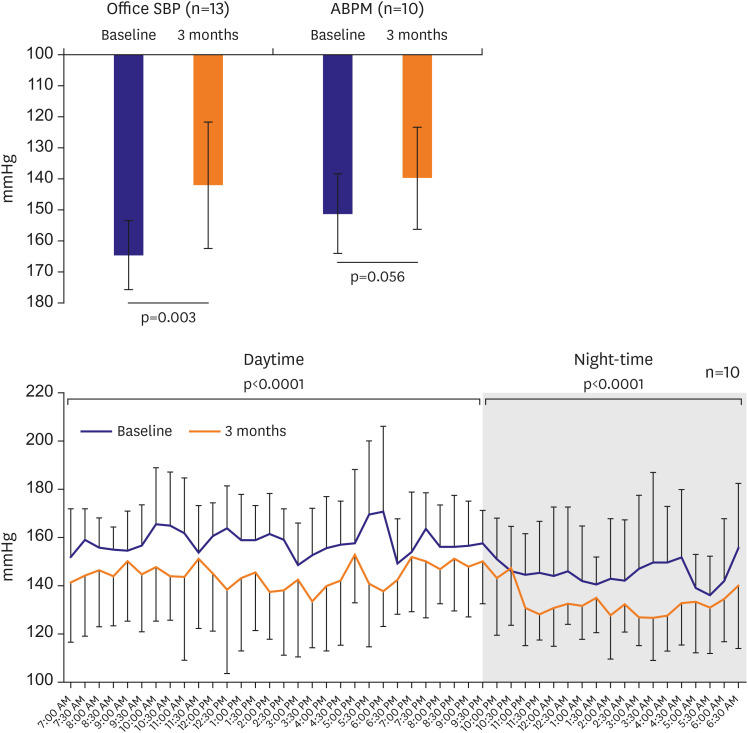
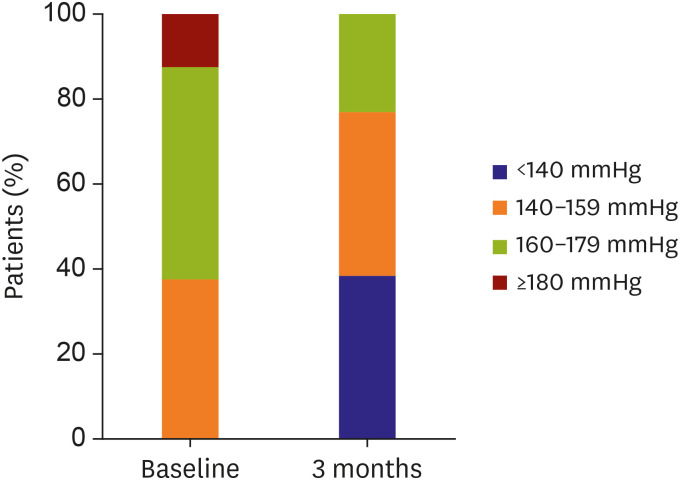
No major adverse events occurred after RDN for 6 month of follow-up period. There was no vascular complication either by CT or duplex ultrasonogram. The office SBP was significantly reduced from 164.6±11.6 mmHg at baseline to 142.0±20.4 mmHg (−24.4±24.4 mmHg, p=0.003) at 3 months. The ambulatory SBP was reduced from 151.44±12.85 mmHg at baseline to 140.0±16.5 mmHg (−13.1±18.9 mmHg, p=0.056) at 3 months.
Conclusion
RDN with the DENEX™ system showed a favorable safety profile in resistant hypertension. A significant reduction in office SBP and a borderline reduction in ambulatory SBP were observed.
Keyword
Figure
Cited by 2 articles
-
Renal Denervation, Come Back Time?
Sang-Ho Jo
Korean Circ J. 2020;51(1):56-57. doi: 10.4070/kcj.2020.0479.Current Status and Future Perspectives of Renal Denervation
Ki Hong Choi, Seung-Hyuk Choi
Korean Circ J. 2021;51(9):717-732. doi: 10.4070/kcj.2021.0175.
Reference
-
1. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018; 36:1953–2041. PMID: 30234752.2. Park S. Ideal target blood pressure in hypertension. Korean Circ J. 2019; 49:1002–1009. PMID: 31646769.
Article3. Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009; 373:1275–1281. PMID: 19332353.
Article4. Symplicity HTN-2 Investigators. Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010; 376:1903–1909. PMID: 21093036.5. Böhm M, Mahfoud F, Ukena C, et al. First report of the Global SYMPLICITY Registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension. 2015; 65:766–774. PMID: 25691618.
Article6. Kim BK, Böhm M, Mahfoud F, et al. Renal denervation for treatment of uncontrolled hypertension in an Asian population: results from the Global SYMPLICITY Registry in South Korea (GSR Korea). J Hum Hypertens. 2016; 30:315–321. PMID: 26155994.
Article7. Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014; 370:1393–1401. PMID: 24678939.
Article8. Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015; 36:219–227. PMID: 25400162.9. Mason E, Tofield A. New analysis of the SYMPLICITY HTN-3 trial. Eur Heart J. 2015; 36:535. PMID: 25884066.10. Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018; 391:2335–2345. PMID: 29803590.11. Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018; 391:2346–2355. PMID: 29803589.12. Tsioufis C, Dimitriadis K, Papademetriou V, Tousoulis D. SPYRAL HTN-OFF MED study: renal denervation in the spiral orbits of current results and future studies. Hellenic J Cardiol. 2017; 58:320–321. PMID: 29056558.
Article13. Worthley SG, Tsioufis CP, Worthley MI, et al. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013; 34:2132–2140. PMID: 23782649.
Article14. Tzafriri AR, Keating JH, Markham PM, et al. Arterial microanatomy determines the success of energy-based renal denervation in controlling hypertension. Sci Transl Med. 2015; 7:285ra65.
Article15. Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017; 390:2160–2170. PMID: 28859944.16. Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020; 395:1444–1451. PMID: 32234534.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status and Future Perspectives of Renal Denervation
- Animal model evaluation of a novel renal denervation system for future laparoscopic treatment of resistant hypertension
- Percutaneous Renal Sympathetic Denervation for the Treatment of Resistant Hypertension with Heart Failure: First Experience in Korea
- Effectiveness of renal denervation in the treatment of hypertension: a literature review
- Renal Denervation for Chronic Heart Failure: Background and Pathophysiological Rationale